Molecular mechanisms regulating aquaporin-2 in kidney collecting duct
- PMID: 27760771
- PMCID: PMC5243240
- DOI: 10.1152/ajprenal.00485.2016
Molecular mechanisms regulating aquaporin-2 in kidney collecting duct
Abstract
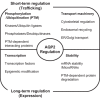
The kidney collecting duct is an important renal tubular segment for regulation of body water homeostasis and urine concentration. Water reabsorption in the collecting duct principal cells is controlled by vasopressin, a peptide hormone that induces the osmotic water transport across the collecting duct epithelia through regulation of water channel proteins aquaporin-2 (AQP2) and aquaporin-3 (AQP3). In particular, vasopressin induces both intracellular translocation of AQP2-bearing vesicles to the apical plasma membrane and transcription of the Aqp2 gene to increase AQP2 protein abundance. The signaling pathways, including AQP2 phosphorylation, RhoA phosphorylation, intracellular calcium mobilization, and actin depolymerization, play a key role in the translocation of AQP2. This review summarizes recent data demonstrating the regulation of AQP2 as the underlying molecular mechanism for the homeostasis of water balance in the body.
Keywords: aquaporin-2; arginine vasopressin; body water balance; intracellular trafficking.
Copyright © 2016 the American Physiological Society.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

