Sustained High Effectiveness of RotaTeq on Hospitalizations Attributable to Rotavirus-Associated Gastroenteritis During 4 Years in Finland
- PMID: 27760800
- PMCID: PMC7107484
- DOI: 10.1093/jpids/piw061
Sustained High Effectiveness of RotaTeq on Hospitalizations Attributable to Rotavirus-Associated Gastroenteritis During 4 Years in Finland
Abstract
Key points: The effectiveness of pentavalent rotavirus vaccine against rotavirus-associated hospitalization was more than 90% 4 years after introduction into the national immunization program in Finland. A major impact on hospitalization for all-cause gastroenteritis was observed also.
Background: Rotavirus vaccination with exclusive use of RotaTeq was added to the National Immunization Programme (NIP) of Finland in September 2009. The objective of our study was to estimate the effectiveness and impact of RotaTeq after 4 years of follow-up.
Methods: Between 2009 and 2013, we conducted a prospective surveillance study of children aged <16 years with acute gastroenteritis (AGE) and admitted in 2 hospitals in Finland. Rotavirus and other gastroenteritis viruses were detected in stool samples by reverse-transcription polymerase chain reaction (RT-PCR) and enzyme-linked immunosorbent assays. The effectiveness of RotaTeq was investigated by using a case-control design; wild-type rotavirus-positive children were classified as "cases" and rotavirus-negative children as "controls." Hospital discharge records were used to estimate the impact of RotaTeq on rotavirus-associated AGE (RV-AGE) and all-cause AGE (AC-AGE) hospitalizations of age-eligible children in the NIP by comparing the prevaccination (2001-2006) and post-NIP seasons (2009-2013).
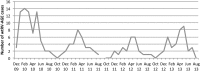
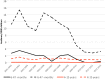
Results: The crude estimate of the effectiveness of RotaTeq to prevent RV-AGE hospitalization in NIP age-eligible children was 94.4% (95% confidence interval, 79.8%-98.4%). No change in prevalent wild-type rotavirus genotypes was observed. Vaccine-derived rotaviruses were detected in 8% of the children with RV-AGE, with a probable causal association in 2 children. Hospital discharge records revealed that RV-AGE and AC-AGE hospitalizations in children aged <16 years decreased in the two post-NIP seasons by 79% and 58%, respectively, compared to those in the prevaccination seasons.
Conclusions: Over 4 years of follow-up, high rotavirus vaccine coverage in the NIP (>95%) has led to a major reduction in RV-AGE and AC-AGE hospitalizations without a resurgence of rotavirus activity. However, rotavirus continues to circulate in older unvaccinated children.
Keywords: RotaTeq; effectiveness; gastroenteritis; impact; rotavirus; vaccine.
© The Author 2016. Published by Oxford University Press on behalf of The Journal of the Pediatric Infectious Diseases Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures



References
-
- Tate JE, Burton AH, Boschi-Pinto C et al. . 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:136–41. - PubMed
-
- Hartwig S, Uhari M, Renko M, Bertet P, Hemming M, Vesikari T. Hospital bed occupancy for rotavirus and all cause acute gastroenteritis in two Finnish hospitals before and after the implementation of the national rotavirus vaccination program with RotaTeq. BMC Health Serv Res 2014; 14:632. - PMC - PubMed
-
- Vesikari T, Karvonen A, Prymula R et al. . Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet 2007; 370:1757–63. - PubMed
-
- Vesikari T, Matson DO, Dennehy P et al. . Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 2006; 354:23–33. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

