Rituximab in multiple sclerosis: A retrospective observational study on safety and efficacy
- PMID: 27760868
- PMCID: PMC5109942
- DOI: 10.1212/WNL.0000000000003331
Rituximab in multiple sclerosis: A retrospective observational study on safety and efficacy
Abstract
Objective: To investigate the safety and efficacy of rituximab in multiple sclerosis (MS).
Methods: In this retrospective uncontrolled observational multicenter study, off-label rituximab-treated patients with MS were identified through the Swedish MS register. Outcome data were collected from the MS register and medical charts. Adverse events (AEs) grades 2-5 according to the Common Terminology Criteria for Adverse Events were recorded.
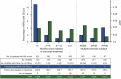
Results: A total of 822 rituximab-treated patients with MS were identified: 557 relapsing-remitting MS (RRMS), 198 secondary progressive MS (SPMS), and 67 primary progressive MS (PPMS). At baseline, 26.2% had contrast-enhancing lesions (CELs). Patients were treated with 500 or 1,000 mg rituximab IV every 6-12 months, during a mean 21.8 (SD 14.3) months. During treatment, the annualized relapse rates were 0.044 (RRMS), 0.038 (SPMS), and 0.015 (PPMS), and 4.6% of patients displayed CELs. Median Expanded Disability Status Scale remained unchanged in RRMS (p = 0.42) and increased by 0.5 and 1.0 in SPMS and PPMS, respectively (p = 0.10 and 0.25). Infusion-related AEs occurred during 7.8% of infusions and most were mild. A total of 89 AEs grades ≥2 (of which 76 infections) were recorded in 72 patients. No case of progressive multifocal leukoencephalopathy was detected.
Conclusions: This is the largest cohort of patients with MS treated with rituximab reported so far. The safety, clinical, and MRI findings in this heterogeneous real-world cohort treated with different doses of rituximab were similar to those reported in previous randomized controlled trials on B-cell depletion therapy in MS.
Classification of evidence: This study provides Class IV evidence that for patients with MS, rituximab is safe and effective.
© 2016 American Academy of Neurology.
Figures


Comment in
-
Rituximab for treating multiple sclerosis: Off-label but on target.Neurology. 2016 Nov 15;87(20):2070-2071. doi: 10.1212/WNL.0000000000003345. Epub 2016 Oct 19. Neurology. 2016. PMID: 27760871 No abstract available.
References
-
- Hauser SL, Waubant E, Arnold DL, et al. . B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med 2008;358:676–688. - PubMed
-
- Hawker K, O'Connor P, Freedman MS, et al. . Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol 2009;66:460–471. - PubMed
-
- Kappos L, Li D, Calabresi PA, et al. . Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet 2011;378:1779–1787. - PubMed
-
- Sorensen PS, Lisby S, Grove R, et al. . Safety and efficacy of ofatumumab in relapsing-remitting multiple sclerosis: a phase 2 study. Neurology 2014;82:573–581. - PubMed
-
- Fyfe I. In the news: ocrelizumab excites ECTRIMS. Nat Rev Neurol 2015;11:667.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
