Dopamine D3 Receptor Antagonists as Potential Therapeutics for the Treatment of Neurological Diseases
- PMID: 27761108
- PMCID: PMC5050208
- DOI: 10.3389/fnins.2016.00451
Dopamine D3 Receptor Antagonists as Potential Therapeutics for the Treatment of Neurological Diseases
Abstract
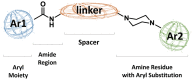
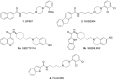
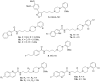
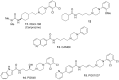
D3 receptors represent a major focus of current drug design and development of therapeutics for dopamine-related pathological states. Their close homology with the D2 receptor subtype makes the development of D3 selective antagonists a challenging task. In this review, we explore the relevance and therapeutic utility of D3 antagonists or partial agonists endowed with multireceptor affinity profile in the field of central nervous system disorders such as schizophrenia and drug abuse. In fact, the peculiar distribution and low brain abundance of D3 receptors make them a valuable target for the development of drugs devoid of motor side effects classically elicited by D2 antagonists. Recent research efforts were devoted to the conception of chemical templates possibly endowed with a multi-target profile, especially with regards to other G-protein-coupled receptors (GPCRs). A comprehensive overview of the recent literature in the field is herein provided. In particular, the evolution of the chemical templates has been tracked, according to the growing advancements in both the structural information and the refinement of the key pharmacophoric elements. The receptor/multireceptor affinity and functional profiles for the examined compounds have been covered, together with their most significant pharmacological applications.
Keywords: GPCR; dopamine; drug optimization; multi-targeting approach; receptor antagonists; selectivity.
Figures

References
-
- Ananthan S., Saini S. K., Zhou G., Hobrath J. V., Padmalayam I., Zhai L., et al. (2014). Design, synthesis, and structure-activity relationship studies of a series of [4-(4-carboxamidobutyl)]-1-arylpiperazines: insights into structural features contributing to dopamine D3 versus D2 receptor subtype selectivity. J. Med. Chem. 57, 7042–7060. 10.1021/jm500801r - DOI - PMC - PubMed
-
- Aragona B. J., Day J. J., Roitman M. F., Cleaveland N. A., Wightman R. M., Carelli R. M. (2009). Regional specificity in the real-time development of phasic dopamine transmission patterns during acquisition of a cue-cocaine association in rats. Eur. J. Neurosci. 30, 1889–1899. 10.1111/j.1460-9568.2009.07027.x - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

