Saccadic and Postsaccadic Disconjugacy in Zebrafish Larvae Suggests Independent Eye Movement Control
- PMID: 27761109
- PMCID: PMC5050213
- DOI: 10.3389/fnsys.2016.00080
Saccadic and Postsaccadic Disconjugacy in Zebrafish Larvae Suggests Independent Eye Movement Control
Abstract
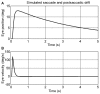
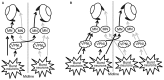
Spontaneous eye movements of zebrafish larvae in the dark consist of centrifugal saccades that move the eyes from a central to an eccentric position and postsaccadic centripetal drifts. In a previous study, we showed that the fitted single-exponential time constants of the postsaccadic drifts are longer in the temporal-to-nasal (T->N) direction than in the nasal-to-temporal (N->T) direction. In the present study, we further report that saccadic peak velocities are higher and saccadic amplitudes are larger in the N->T direction than in the T->N direction. We investigated the underlying mechanism of this ocular disconjugacy in the dark with a top-down approach. A mathematic ocular motor model, including an eye plant, a set of burst neurons and a velocity-to-position neural integrator (VPNI), was built to simulate the typical larval eye movements in the dark. The modeling parameters, such as VPNI time constants, neural impulse signals generated by the burst neurons and time constants of the eye plant, were iteratively adjusted to fit the average saccadic eye movement. These simulations suggest that four pools of burst neurons and four pools of VPNIs are needed to explain the disconjugate eye movements in our results. A premotor mechanism controls the synchronous timing of binocular saccades, but the pools of burst and integrator neurons in zebrafish larvae seem to be different (and maybe separate) for both eyes and horizontal directions, which leads to the observed ocular disconjugacies during saccades and postsaccadic drifts in the dark.
Keywords: brainstem; gaze holding; larvae; ocular motor; saccades; zebrafish.
Figures



Similar articles
-
Visually induced adaptive changes in primate saccadic oculomotor control signals.J Neurophysiol. 1985 Oct;54(4):940-58. doi: 10.1152/jn.1985.54.4.940. J Neurophysiol. 1985. PMID: 4067628
-
Horizontal saccade disconjugacy in strabismic monkeys.Invest Ophthalmol Vis Sci. 2007 Jul;48(7):3107-14. doi: 10.1167/iovs.06-0955. Invest Ophthalmol Vis Sci. 2007. PMID: 17591880 Free PMC article.
-
Short-latency disparity vergence responses and their dependence on a prior saccadic eye movement.J Neurophysiol. 1996 Apr;75(4):1392-410. doi: 10.1152/jn.1996.75.4.1392. J Neurophysiol. 1996. PMID: 8727386
-
Eye-head coordination in labyrinthine-defective humans.Exp Brain Res. 1998 Oct;122(3):260-74. doi: 10.1007/s002210050514. Exp Brain Res. 1998. PMID: 9808299
-
Signal transformations required for the generation of saccadic eye movements.Annu Rev Neurosci. 1990;13:309-36. doi: 10.1146/annurev.ne.13.030190.001521. Annu Rev Neurosci. 1990. PMID: 2183679 Review.
Cited by
-
Fourier Motion Processing in the Optic Tectum and Pretectum of the Zebrafish Larva.Front Neural Circuits. 2022 Jan 7;15:814128. doi: 10.3389/fncir.2021.814128. eCollection 2021. Front Neural Circuits. 2022. PMID: 35069128 Free PMC article.
-
Zebrafish dscaml1 Deficiency Impairs Retinal Patterning and Oculomotor Function.J Neurosci. 2020 Jan 2;40(1):143-158. doi: 10.1523/JNEUROSCI.1783-19.2019. Epub 2019 Nov 4. J Neurosci. 2020. PMID: 31685652 Free PMC article.
-
Dog eye movements are slower than human eye movements.J Eye Mov Res. 2020 Feb 5;12(8):10.16910/jemr.12.8.4. doi: 10.16910/jemr.12.8.4. J Eye Mov Res. 2020. PMID: 33828775 Free PMC article.
-
A distributed saccade-associated network encodes high velocity conjugate and monocular eye movements in the zebrafish hindbrain.Sci Rep. 2021 Jun 16;11(1):12644. doi: 10.1038/s41598-021-90315-2. Sci Rep. 2021. PMID: 34135354 Free PMC article.
-
Functional architecture underlying binocular coordination of eye position and velocity in the larval zebrafish hindbrain.BMC Biol. 2019 Dec 29;17(1):110. doi: 10.1186/s12915-019-0720-y. BMC Biol. 2019. PMID: 31884959 Free PMC article.
References
-
- Aksay E., Baker R., Seung H. S., Tank D. W. (2000). Anatomy and discharge properties of pre-motor neurons in the goldfish medulla that have eye-position signals during fixations. J. Neurophysiol. 84, 1035–1049. - PubMed
-
- Cannon S. C., Robinson D. A. (1987). Loss of the neural integrator of the oculomotor system from brain stem lesions in monkey. J. Neurophysiol. 57, 1383–1409. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

