Catch me if you can: Leukemia Escape after CD19-Directed T Cell Immunotherapies
- PMID: 27761200
- PMCID: PMC5061074
- DOI: 10.1016/j.csbj.2016.09.003
Catch me if you can: Leukemia Escape after CD19-Directed T Cell Immunotherapies
Abstract
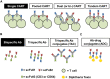
Immunotherapy is the revolution in cancer treatment of this last decade. Among multiple approaches able to harness the power of the immune system against cancer, T cell based immunotherapies represent one of the most successful examples. In particular, biotechnological engineering of protein structures, like the T cell receptor or the immunoglobulins, allowed the generation of synthetic peptides like chimeric antigen receptors and bispecific antibodies that are able to redirect non-tumor specific T cells to recognize and kill leukemic cells. The anti-CD19/CD3 bispecific antibody blinatumomab and anti-CD19 chimeric antigen receptor T cells (CART19) have produced deep responses in patients with relapsed and refractory B-cell acute leukemias. However, although the majority of these patients responds to anti-CD19 immunotherapy, a subset of them still relapses. Interestingly, a novel family of leukemia escape mechanisms has been described, all characterized by the apparent loss of CD19 on the surface of leukemic blasts. This extraordinary finding demonstrates the potent selective pressure of CART19/blinatumomab that drives extreme and specific escape strategies by leukemic blasts. Patients with CD19-negative relapsed leukemia have very poor prognosis and novel approaches to treat and ideally prevent antigen-loss are direly needed. In this review we discuss the incidence, mechanisms and therapeutic approaches for CD19-negative leukemia relapses occuring after CD19-directed T cell immunotherapies and present our future perspective.
Keywords: Adoptive cell therapy; Bispecific antibodies; Blinatumomab; CART19; CTL019; Chimeric antigen receptor T cells; Leukemia; biCAR; dualCART; tanCAR.
Figures
References
-
- Ruella M., Kalos M. Adoptive immunotherapy for cancer. Immunol Rev. 2014;257(1):14–38. - PubMed
-
- Ruella M., June C.H. Chimeric antigen receptor T cells for B cell neoplasms: choose the right CAR for you. Curr Hematol Malig Rep. 2016 - PubMed
-
- Ruella M., Gill S. How to train your T cell: genetically engineered chimeric antigen receptor T cells versus bispecific T-cell engagers to target CD19 in B acute lymphoblastic leukemia. Expert Opin Biol Ther. 2015;15(6):761–766. - PubMed
-
- Przepiorka D., Ko C.W., Deisseroth A., Yancey C.L., Candau-Chacon R., Chiu H.J. FDA approval: blinatumomab. Clin Cancer Res. 2015;21(18):4035–4039. - PubMed
-
- Haas C., Krinner E., Brischwein K., Hoffmann P., Lutterbuse R., Schlereth B. Mode of cytotoxic action of T cell-engaging BiTE antibody MT110. Immunobiology. 2009;214(6):441–453. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
