Idiopathic sclerosing orbital inflammation mimicking a malignant spindle cell tumor in a dog
- PMID: 27761242
- PMCID: PMC5054466
- DOI: 10.1002/ccr3.639
Idiopathic sclerosing orbital inflammation mimicking a malignant spindle cell tumor in a dog
Abstract
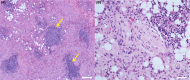
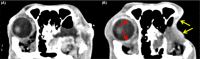
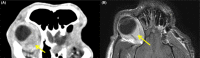
A dog presented with a retrobulbar mass, diagnosed histopathologically as malignant spindle cell neoplasia. Emergence of analogous findings in the contralateral orbit prompted extended immunohistochemistry of the original mass and reassignment to idiopathic sclerosing orbital inflammation. Early incisional biopsy with extended immunohistochemical analysis should be considered for canine orbital tumors.
Keywords: Case report; IgG4; exophthalmos; idiopathic orbital inflammation; lymphoplasmacytic infiltration; magnetic resonance imaging; radiation therapy; retrobulbar.
Figures


References
-
- Knight, C. , Fan E., and Riis R.. 2009. Inflammatory myofibroblastic tumors in two dogs. Vet. Pathol. 46:273–276. - PubMed
-
- Miller, S. A. , Van der Woerdt A., and Bartick T. E.. 2000. Retrobulbar pseudotumor of the orbit in a cat. J. Am. Vet. Med. Assoc. 216:356–358. - PubMed
-
- LaDue, T. , Klein M. K., and Veterinary Radiation Therapy Oncology Group . 2001. Toxicity criteria of the veterinary radiation therapy oncology group. Vet. Radiol. Ultrasound. 42:475–476. - PubMed
-
- Chow, S. P. , Nastri A., and Hardy T.. 2010. Infratemporal inflammatory myofibroblastic tumour with orbital extension. Clin. Experiment Ophthalmol. 38:727–730. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

