Metformin Inhibits Advanced Glycation End Products-Induced Inflammatory Response in Murine Macrophages Partly through AMPK Activation and RAGE/NF κ B Pathway Suppression
- PMID: 27761470
- PMCID: PMC5059570
- DOI: 10.1155/2016/4847812
Metformin Inhibits Advanced Glycation End Products-Induced Inflammatory Response in Murine Macrophages Partly through AMPK Activation and RAGE/NF κ B Pathway Suppression
Abstract
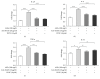
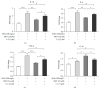
Advanced glycation end products (AGEs) are major inflammatory mediators in diabetes, affecting atherosclerosis progression via macrophages. Metformin slows diabetic atherosclerosis progression through mechanisms that remain to be fully elucidated. The present study of murine bone marrow derived macrophages showed that (1) AGEs enhanced proinflammatory cytokines (interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α (TNF-α)) mRNA expression, RAGE expression, and NFκB activation; (2) metformin pretreatment inhibited AGEs effects and AGEs-induced cluster designation 86 (CD86) (M1 marker) expression, while promoting CD206 (M2 marker) surface expression and anti-inflammatory cytokine (IL-10) mRNA expression; and (3) the AMPK inhibitor, Compound C, attenuated metformin effects. In conclusion, metformin inhibits AGEs-induced inflammatory response in murine macrophages partly through AMPK activation and RAGE/NFκB pathway suppression.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

