Immunology of neuromyelitis optica during pregnancy
- PMID: 27761482
- PMCID: PMC5056648
- DOI: 10.1212/NXI.0000000000000288
Immunology of neuromyelitis optica during pregnancy
Abstract
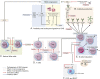
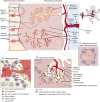
Anti-aquaporin-4 (AQP4) autoantibody plays a key role in the pathogenesis of neuromyelitis optica (NMO). Studies have shown increased relapse rates in patients with NMO during pregnancy and postpartum. High estrogen levels during pregnancy can increase activation-induced cytidine deaminase expression, which is responsible for immunoglobulin production. Additionally, sex hormones may influence antibody glycosylation, with effects on antibody function. Estrogen decreases apoptosis of self-reactive B cells, through upregulation of antiapoptotic molecules. Furthermore, high estrogen levels during pregnancy can boost B-cell activating factor and type 1 interferon (IFN) production, facilitating development of self-reactive peripheral B cells in association with increased disease activity. Elevated levels of estrogen during pregnancy decrease IFN-γ generation, which causes a shift toward T helper (Th) 2 immunity, thereby propagating NMO pathogenesis. Women with NMO have an elevated rate of pregnancy complications including miscarriage and preeclampsia, which are associated with increased Th17 cells and reduction of T-regulatory cells. These in turn can enhance inflammation in NMO. Increased regulatory natural killer cells (CD56-) during pregnancy can enhance Th2-mediated immunity, thereby increasing inflammation. In the placenta, trophoblasts express AQP4 antigen and are exposed to maternal blood containing anti-AQP4 antibodies. Animal models have shown that anti-AQP4 antibodies can bind to AQP4 antigen in placenta leading to complement deposition and placental necrosis. Reduction of regulatory complements has been associated with placental insufficiency, and it is unclear whether these are altered in NMO. Further studies are required to elucidate the specific mechanisms of disease worsening, as well as the increased rate of complications during pregnancy in women with NMO.
Figures

References
-
- Lennon VA, Wingerchuk DM, Kryzer TJ, et al. . A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 2004;364:2106–2112. - PubMed
-
- Wang HH, Dai YQ, Qiu W, et al. . Interleukin-17-secreting T cells in neuromyelitis optica and multiple sclerosis during relapse. J Clin Neurosci 2011;18:1313–1317. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
