The Largest Response Component in the Motor Cortex Reflects Movement Timing but Not Movement Type
- PMID: 27761519
- PMCID: PMC5069299
- DOI: 10.1523/ENEURO.0085-16.2016
The Largest Response Component in the Motor Cortex Reflects Movement Timing but Not Movement Type
Abstract
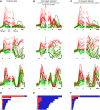
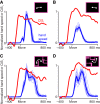
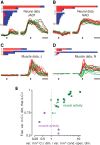
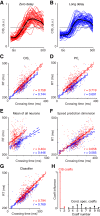
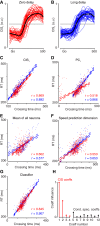
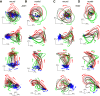
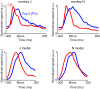
Neural activity in monkey motor cortex (M1) and dorsal premotor cortex (PMd) can reflect a chosen movement well before that movement begins. The pattern of neural activity then changes profoundly just before movement onset. We considered the prediction, derived from formal considerations, that the transition from preparation to movement might be accompanied by a large overall change in the neural state that reflects when movement is made rather than which movement is made. Specifically, we examined "components" of the population response: time-varying patterns of activity from which each neuron's response is approximately composed. Amid the response complexity of individual M1 and PMd neurons, we identified robust response components that were "condition-invariant": their magnitude and time course were nearly identical regardless of reach direction or path. These condition-invariant response components occupied dimensions orthogonal to those occupied by the "tuned" response components. The largest condition-invariant component was much larger than any of the tuned components; i.e., it explained more of the structure in individual-neuron responses. This condition-invariant response component underwent a rapid change before movement onset. The timing of that change predicted most of the trial-by-trial variance in reaction time. Thus, although individual M1 and PMd neurons essentially always reflected which movement was made, the largest component of the population response reflected movement timing rather than movement type.
Keywords: condition-invariant signal; dPCA; movement initiation; movement triggering; reaction time; state space.
Conflict of interest statement
The authors report no conflict of interest.
Figures
References
-
- Bastian A, Schöner G, Riehle A (2003) Preshaping and continuous evolution of motor cortical representations during movement preparation. Eur J Neurosci 18:2047–2058. - PubMed
-
- Brendel W, Romo R, Machens C (2011) Demixed principal component analysis. Adv Neural Info Process Syst 24:1–9.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
