The role of perivascular adipose tissue in obesity-induced vascular dysfunction
- PMID: 27761903
- PMCID: PMC5610151
- DOI: 10.1111/bph.13650
The role of perivascular adipose tissue in obesity-induced vascular dysfunction
Abstract
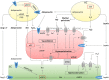
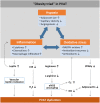
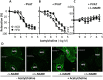
Under physiological conditions, perivascular adipose tissue (PVAT) attenuates agonist-induced vasoconstriction by releasing vasoactive molecules including hydrogen peroxide, angiotensin 1-7, adiponectin, methyl palmitate, hydrogen sulfide, NO and leptin. This anticontractile effect of PVAT is lost under conditions of obesity. The central mechanism underlying this PVAT dysfunction in obesity is likely to be an 'obesity triad' (consisting of PVAT hypoxia, inflammation and oxidative stress) that leads to the impairment of PVAT-derived vasoregulators. The production of hydrogen sulfide, NO and adiponectin by PVAT is reduced in obesity, whereas the vasodilator response to leptin is impaired (vascular leptin resistance). Strikingly, the vasodilator response to acetylcholine is reduced only in PVAT-containing, but not in PVAT-free thoracic aorta isolated from diet-induced obese mice, indicating a unique role for PVAT in obesity-induced vascular dysfunction. Furthermore, PVAT dysfunction has also been observed in small arteries isolated from the gluteal/visceral fat biopsy samples of obese individuals. Therefore, PVAT may represent a new therapeutic target for vascular complications in obesity. A number of approaches are currently being tested under experimental conditions. Potential therapeutic strategies improving PVAT function include body weight reduction, enhancing PVAT hydrogen sulfide release (e.g. rosiglitazone, atorvastatin and cannabinoid CB1 receptor agonists) and NO production (e.g. arginase inhibitors), inhibition of the renin-angiotensin-aldosterone system, inhibition of inflammation with melatonin or cytokine antagonists, activators of AMP-activated kinase (e.g. metformin, resveratrol and diosgenin) and adiponectin releasers or expression enhancers.
Linked articles: This article is part of a themed section on Molecular Mechanisms Regulating Perivascular Adipose Tissue - Potential Pharmacological Targets? To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.20/issuetoc.
© 2016 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Figures
References
-
- Agabiti‐Rosei C, De Ciuceis C, Rossini C, Porteri E, Rodella LF, Withers SB et al. (2014). Anticontractile activity of perivascular fat in obese mice and the effect of long‐term treatment with melatonin. J Hypertens 32: 1264–1274. - PubMed
-
- Aghamohammadzadeh R, Greenstein AS, Yadav R, Jeziorska M, Hama S, Soltani F et al. (2013). Effects of bariatric surgery on human small artery function: evidence for reduction in perivascular adipocyte inflammation, and the restoration of normal anticontractile activity despite persistent obesity. J Am Coll Cardiol 62: 128–135. - PMC - PubMed
-
- Ahmed SR, Johansson BL, Karlsson MG, Souza DS, Dashwood MR, Loesch A (2004). Human saphenous vein and coronary bypass surgery: ultrastructural aspects of conventional and “no‐touch” vein graft preparations. Histol Histopathol 19: 421–433. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

