Protease induced plasticity: matrix metalloproteinase-1 promotes neurostructural changes through activation of protease activated receptor 1
- PMID: 27762280
- PMCID: PMC5071868
- DOI: 10.1038/srep35497
Protease induced plasticity: matrix metalloproteinase-1 promotes neurostructural changes through activation of protease activated receptor 1
Abstract
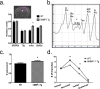
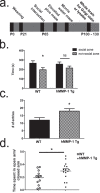
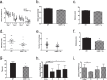
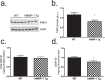
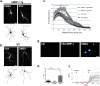
Matrix metalloproteinases (MMPs) are a family of secreted endopeptidases expressed by neurons and glia. Regulated MMP activity contributes to physiological synaptic plasticity, while dysregulated activity can stimulate injury. Disentangling the role individual MMPs play in synaptic plasticity is difficult due to overlapping structure and function as well as cell-type specific expression. Here, we develop a novel system to investigate the selective overexpression of a single MMP driven by GFAP expressing cells in vivo. We show that MMP-1 induces cellular and behavioral phenotypes consistent with enhanced signaling through the G-protein coupled protease activated receptor 1 (PAR1). Application of exogenous MMP-1, in vitro, stimulates PAR1 dependent increases in intracellular Ca2+ concentration and dendritic arborization. Overexpression of MMP-1, in vivo, increases dendritic complexity and induces biochemical and behavioral endpoints consistent with increased GPCR signaling. These data are exciting because we demonstrate that an astrocyte-derived protease can influence neuronal plasticity through an extracellular matrix independent mechanism.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

