Comparative Study of the Catalytic Activities of Three Distinct Carbonaceous Materials through Photocatalytic Oxidation, CO Conversion, Dye Degradation, and Electrochemical Measurements
- PMID: 27762289
- PMCID: PMC5071860
- DOI: 10.1038/srep35500
Comparative Study of the Catalytic Activities of Three Distinct Carbonaceous Materials through Photocatalytic Oxidation, CO Conversion, Dye Degradation, and Electrochemical Measurements
Abstract
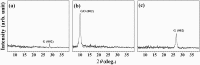
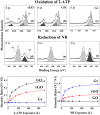
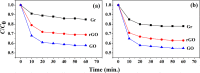
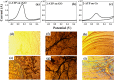
In order to compare the catalytic activities of reduced graphene oxide (rGO), graphene oxide (GO), and graphene, we conducted oxidation of 2-aminothiophenol (2-ATP) and reduction of nitrobenzene (NB) in their presence by using high-resolution photoemission spectroscopy (HRPES). In addition, we determined conversion rates of CO to CO2 in the presence of these catalysts by performing a residual gas analyzer (RGA) under a UHV condition, Orange II and methylene blue degradations UV-vis spectrophotometry, and electrochemistry (EC) measurements in an aqueous solution, as well as by obtaining cyclic voltammograms and determining the change of the condition of electrodes before and after the oxidation of 2-ATP. We found that we can successively fabricate GO (oxidation) and graphene (reduction) from rGO by controlling the oxidation or reduction procedure time and then clearly comparing the critical properties among them as we perform various oxidation and reduction activities.
Figures



References
-
- Lomeda J. R., Doyle C. D., Kosynkin D. V., Hwang W.-F. & Tour J. M. Diazonium Functionalization of Surfactant-Wrapped Chemically Converted Graphene Sheets. J. Am. Chem. Soc. 130, 16201–16206 (2008). - PubMed
-
- Chen D., Zhang H., Liua Y. & Li, J. Graphene and its derivatives for the development of solar cells, photoelectrochemical, and photocatalytic applications. Energy & Environmental Science 6, 1362–1387 (2013).
-
- Hu P. & Long M. Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and applications. Applied Catalysis B: Environmental 181, 103–117 (2016).
-
- Tan Y. B. & Lee J.-M. Graphene for supercapacitor applications. Journal of Materials Chemistry A 1, 14814–14843 (2013).
-
- Dreyer D. R., Jia H.-P. & Bielawski C. W. Graphene Oxide: A Convenient Carbocatalyst for Facilitating Oxidation and Hydration Reactions. Angew. Chem. Int. Ed. 122(38), 6965–6968 (2010). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

