Antigen-affinity controls pre-germinal center B cell selection by promoting Mcl-1 induction through BAFF receptor signaling
- PMID: 27762293
- PMCID: PMC5071843
- DOI: 10.1038/srep35673
Antigen-affinity controls pre-germinal center B cell selection by promoting Mcl-1 induction through BAFF receptor signaling
Erratum in
-
Erratum: Antigen-affinity controls pre-germinal center B cell selection by promoting Mcl-1 induction through BAFF receptor signaling.Sci Rep. 2017 Jan 12;7:39366. doi: 10.1038/srep39366. Sci Rep. 2017. PMID: 28079103 Free PMC article. No abstract available.
Abstract
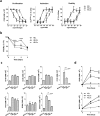
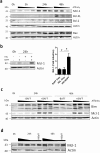
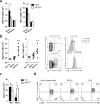
Upon antigen encounter, the responsive B cell pool undergoes stringent selection which eliminates cells with low B cell receptor (BCR) affinity. Already before formation of the germinal center, activated B cells of low-affinity are negatively selected in a process that is molecularly not well understood. In this study, we investigated the mechanism behind pre-GC affinity-mediated B cell selection. We applied affinity mutants of HEL antigen and found that rapidly after activation B cells become highly dependent on the cytokine BAFF. Moreover, expression of BAFF receptor CD268 is regulated in a BCR-affinity dependent fashion. High affinity responses via BAFF correlated with PI3K activation, which controlled expression of the pro-survival protein Mcl-1, and thereby increased survival. In the presence of excess BAFF, or in absence of the Mcl-1 antagonist Noxa, more low-affinity B cells survived the first two days after antigen encounter. This resulted in increased numbers of antigen-specific B cells of low affinity upon immunization and reduced the overall affinity of cells that contributed to the germinal center reaction. Our findings elucidate a crucial molecular pathway of B cell selection in the earliest phases of activation by identifying a novel link between BCR affinity and BAFF-R signaling towards Mcl-1.
Figures




Similar articles
-
mTOR-Dependent and Independent Survival Signaling by PI3K in B Lymphocytes.PLoS One. 2016 Jan 19;11(1):e0146955. doi: 10.1371/journal.pone.0146955. eCollection 2016. PLoS One. 2016. PMID: 26785352 Free PMC article.
-
Normal induction but attenuated progression of germinal center responses in BAFF and BAFF-R signaling-deficient mice.J Exp Med. 2003 Oct 20;198(8):1157-69. doi: 10.1084/jem.20030495. Epub 2003 Oct 13. J Exp Med. 2003. PMID: 14557413 Free PMC article.
-
SYK inhibition thwarts the BAFF - B-cell receptor crosstalk and thereby antagonizes Mcl-1 in chronic lymphocytic leukemia.Haematologica. 2017 Nov;102(11):1890-1900. doi: 10.3324/haematol.2017.170571. Epub 2017 Aug 24. Haematologica. 2017. PMID: 28838991 Free PMC article.
-
BAFF and BAFF-Receptor in B Cell Selection and Survival.Front Immunol. 2018 Oct 8;9:2285. doi: 10.3389/fimmu.2018.02285. eCollection 2018. Front Immunol. 2018. PMID: 30349534 Free PMC article. Review.
-
BAFF and the plasticity of peripheral B cell tolerance.Curr Opin Immunol. 2008 Apr;20(2):158-61. doi: 10.1016/j.coi.2008.03.015. Epub 2008 May 2. Curr Opin Immunol. 2008. PMID: 18456486 Free PMC article. Review.
Cited by
-
Hyperactivated PI3Kδ promotes self and commensal reactivity at the expense of optimal humoral immunity.Nat Immunol. 2018 Sep;19(9):986-1000. doi: 10.1038/s41590-018-0182-3. Epub 2018 Aug 20. Nat Immunol. 2018. PMID: 30127432 Free PMC article.
-
B cell-activating factor modulates the factor VIII immune response in hemophilia A.J Clin Invest. 2021 Apr 15;131(8):e142906. doi: 10.1172/JCI142906. J Clin Invest. 2021. PMID: 33651716 Free PMC article. Clinical Trial.
-
Altered B cell signalling in autoimmunity.Nat Rev Immunol. 2017 Jul;17(7):421-436. doi: 10.1038/nri.2017.24. Epub 2017 Apr 10. Nat Rev Immunol. 2017. PMID: 28393923 Free PMC article. Review.
-
Causal Effects of Immune Cells on Reproductive Ill-Health, Including Abnormal Spermatozoa, Polycystic Ovary Syndrome and Spontaneous Abortion: Mendelian Randomization Analyses.J Multidiscip Healthc. 2025 Jun 6;18:3219-3232. doi: 10.2147/JMDH.S524949. eCollection 2025. J Multidiscip Healthc. 2025. PMID: 40496872 Free PMC article.
-
B Cell Competition for Restricted T Cell Help Suppresses Rare-Epitope Responses.Cell Rep. 2018 Oct 9;25(2):321-327.e3. doi: 10.1016/j.celrep.2018.09.029. Cell Rep. 2018. PMID: 30304673 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

