AKT/GSK3β signaling pathway is critically involved in human pluripotent stem cell survival
- PMID: 27762303
- PMCID: PMC5071844
- DOI: 10.1038/srep35660
AKT/GSK3β signaling pathway is critically involved in human pluripotent stem cell survival
Abstract
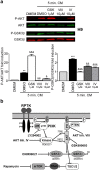
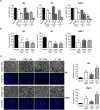
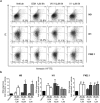
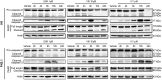
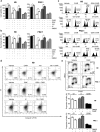
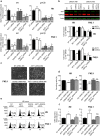
Human embryonic and induced pluripotent stem cells are self-renewing pluripotent stem cells (PSC) that can differentiate into a wide range of specialized cells. Basic fibroblast growth factor is essential for PSC survival, stemness and self-renewal. PI3K/AKT pathway regulates cell viability and apoptosis in many cell types. Although it has been demonstrated that PI3K/AKT activation by bFGF is relevant for PSC stemness maintenance its role on PSC survival remains elusive. In this study we explored the molecular mechanisms involved in the regulation of PSC survival by AKT. We found that inhibition of AKT with three non-structurally related inhibitors (GSK690693, AKT inhibitor VIII and AKT inhibitor IV) decreased cell viability and induced apoptosis. We observed a rapid increase in phosphatidylserine translocation and in the extent of DNA fragmentation after inhibitors addition. Moreover, abrogation of AKT activity led to Caspase-9, Caspase-3, and PARP cleavage. Importantly, we demonstrated by pharmacological inhibition and siRNA knockdown that GSK3β signaling is responsible, at least in part, of the apoptosis triggered by AKT inhibition. Moreover, GSK3β inhibition decreases basal apoptosis rate and promotes PSC proliferation. In conclusion, we demonstrated that AKT activation prevents apoptosis, partly through inhibition of GSK3β, and thus results relevant for PSC survival.
Figures

References
-
- Thomson J. A. et al. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 (1998). - PubMed
-
- Takahashi K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007). - PubMed
-
- Bottcher R. T. & Niehrs C. Fibroblast growth factor signaling during early vertebrate development. Endocr. Rev. 26, 63–77 (2005). - PubMed
-
- Dvorak P. et al. Expression and potential role of fibroblast growth factor 2 and its receptors in human embryonic stem cells. Stem Cells 23, 1200–1211 (2005). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

