Maporal Hantavirus Causes Mild Pathology in Deer Mice (Peromyscus maniculatus)
- PMID: 27763552
- PMCID: PMC5086618
- DOI: 10.3390/v8100286
Maporal Hantavirus Causes Mild Pathology in Deer Mice (Peromyscus maniculatus)
Abstract
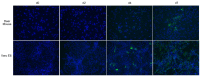
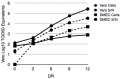
Rodent-borne hantaviruses can cause two human diseases with many pathological similarities: hantavirus cardiopulmonary syndrome (HCPS) in the western hemisphere and hemorrhagic fever with renal syndrome in the eastern hemisphere. Each virus is hosted by specific reservoir species without conspicuous disease. HCPS-causing hantaviruses require animal biosafety level-4 (ABSL-4) containment, which substantially limits experimental research of interactions between the viruses and their reservoir hosts. Maporal virus (MAPV) is a South American hantavirus not known to cause disease in humans, thus it can be manipulated under ABSL-3 conditions. The aim of this study was to develop an ABSL-3 hantavirus infection model using the deer mouse (Peromyscus maniculatus), the natural reservoir host of Sin Nombre virus (SNV), and a virus that is pathogenic in another animal model to examine immune response of a reservoir host species. Deer mice were inoculated with MAPV, and viral RNA was detected in several organs of all deer mice during the 56 day experiment. Infected animals generated both nucleocapsid-specific and neutralizing antibodies. Histopathological lesions were minimal to mild with the peak of the lesions detected at 7-14 days postinfection, mainly in the lungs, heart, and liver. Low to modest levels of cytokine gene expression were detected in spleens and lungs of infected deer mice, and deer mouse primary pulmonary cells generated with endothelial cell growth factors were susceptible to MAPV with viral RNA accumulating in the cellular fraction compared to infected Vero cells. Most features resembled that of SNV infection of deer mice, suggesting this model may be an ABSL-3 surrogate for studying the host response of a New World hantavirus reservoir.
Keywords: Maporal virus; deer mice; hantavirus; reservoir; zoonoses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
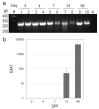
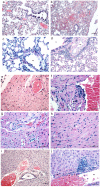
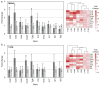
References
-
- Johnson K.M. Hantaviruses: History and overview. Curr. Top. Microbiol. Immunol. 2001;256:1–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

