4EBP-Dependent Signaling Supports West Nile Virus Growth and Protein Expression
- PMID: 27763553
- PMCID: PMC5086619
- DOI: 10.3390/v8100287
4EBP-Dependent Signaling Supports West Nile Virus Growth and Protein Expression
Abstract
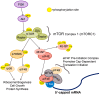
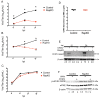
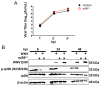
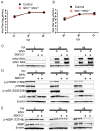
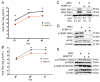
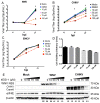
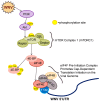
West Nile virus (WNV) is a (+) sense, single-stranded RNA virus in the Flavivirus genus. WNV RNA possesses an m7GpppNm 5' cap with 2'-O-methylation that mimics host mRNAs preventing innate immune detection and allowing the virus to translate its RNA genome through the utilization of cap-dependent translation initiation effectors in a wide variety of host species. Our prior work established the requirement of the host mammalian target of rapamycin complex 1 (mTORC1) for optimal WNV growth and protein expression; yet, the roles of the downstream effectors of mTORC1 in WNV translation are unknown. In this study, we utilize gene deletion mutants in the ribosomal protein kinase called S6 kinase (S6K) and eukaryotic translation initiation factor 4E-binding protein (4EBP) pathways downstream of mTORC1 to define the role of mTOR-dependent translation initiation signals in WNV gene expression and growth. We now show that WNV growth and protein expression are dependent on mTORC1 mediated-regulation of the eukaryotic translation initiation factor 4E-binding protein/eukaryotic translation initiation factor 4E-binding protein (4EBP/eIF4E) interaction and eukaryotic initiation factor 4F (eIF4F) complex formation to support viral growth and viral protein expression. We also show that the canonical signals of mTORC1 activation including ribosomal protein s6 (rpS6) and S6K phosphorylation are not required for WNV growth in these same conditions. Our data suggest that the mTORC1/4EBP/eIF4E signaling axis is activated to support the translation of the WNV genome.
Keywords: RNA; West Nile virus; protein synthesis; translation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Szretter K.J., Daniels B.P., Cho H., Gainey M.D., Yokoyama W.M., Gale M., Jr., Virgin H.W., Klein R.S., Sen G.C., Diamond M.S. 2′-O methylation of the viral mRNA cap by West Nile virus evades IFIT1-dependent and -independent mechanisms of host restriction in vivo. PLoS Pathog. 2012;8:287. doi: 10.1371/journal.ppat.1002698. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

