Measurement characteristics of the childhood Asthma-Control Test and a shortened, child-only version
- PMID: 27763622
- PMCID: PMC5072391
- DOI: 10.1038/npjpcrm.2016.75
Measurement characteristics of the childhood Asthma-Control Test and a shortened, child-only version
Abstract
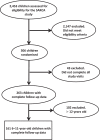
The childhood Asthma-Control Test (C-ACT) is validated for assessing asthma control in paediatric asthma. Among children aged 4-11 years, the C-ACT requires the simultaneous presence of both parent and child. There is an unmet need for a tool that can be used to assess asthma control in children when parents or caregivers are not present such as in the school setting. We assessed the psychometric properties and estimated the minimally important difference (MID) of the C-ACT and a modified version, comprising only the child responses (C-ACTc). Asthma patients aged 6-11 years (n=161) from a previously completed multicenter randomised trial were included. Demographic information, spirometry and questionnaire scores were obtained at baseline and during follow-up. Participants or their guardians kept a daily asthma diary. Internal consistency reliabilities of the C-ACT and C-ACTc were 0.76 and 0.67 (Cronbach's α), respectively. Test-retest reliabilities of the C-ACT and C-ACTc were 0.72 and 0.66 (intra-class correlation), respectively. Significant correlations were noted between C-ACT scores and ACQ scores (Spearman's correlation r=-0.56, 95% CI (-0.66, -0.44), P<0.001). The strength of the correlation between C-ACTc scores and ACQ scores was weaker (Spearman's correlation r=-0.46, 95% CI (-0.58, -0.33), P<0.001). We estimated the MID for the C-ACT and C-ACTc to be 2 points and 1 point, respectively. Among asthma patients aged 6-11 years, the C-ACT had good psychometric properties. The psychometric properties of a shortened child-only version (C-ACTc), although acceptable, are not as strong.
Similar articles
-
Psychometric evaluation of the Brazilian version of the pediatric asthma control and communication instrument.Pediatr Pulmonol. 2020 Aug;55(8):1900-1907. doi: 10.1002/ppul.24851. Epub 2020 May 25. Pediatr Pulmonol. 2020. PMID: 32450011
-
Validation of the Brazilian version of the childhood asthma control test (c-ACT).Pediatr Pulmonol. 2016 Apr;51(4):358-63. doi: 10.1002/ppul.23318. Epub 2015 Sep 30. Pediatr Pulmonol. 2016. PMID: 26422330
-
Translation and validation of the Test of Adherence to Inhalers (TAI) questionnaire among adult patients with asthma in Malaysia.J Asthma. 2021 Sep;58(9):1229-1236. doi: 10.1080/02770903.2020.1776728. Epub 2020 Jun 18. J Asthma. 2021. PMID: 32493083
-
Proxy-reported questionnaires for young children with asthma: a structured review.Eur Respir J. 2013 Aug;42(2):513-26. doi: 10.1183/09031936.00052112. Epub 2012 Nov 8. Eur Respir J. 2013. PMID: 23143547 Review.
-
Asthma control in the quality of life levels of asthmatic patients' caregivers: a systematic review with meta-analysis and meta-regression.J Pediatr (Rio J). 2019 Jul-Aug;95(4):401-409. doi: 10.1016/j.jped.2018.10.010. Epub 2018 Dec 9. J Pediatr (Rio J). 2019. PMID: 30540924
Cited by
-
The home air in agriculture pediatric intervention (HAPI) trial: Rationale and methods.Contemp Clin Trials. 2020 Sep;96:106085. doi: 10.1016/j.cct.2020.106085. Epub 2020 Jul 25. Contemp Clin Trials. 2020. PMID: 32721578 Free PMC article.
-
Yoga Education Program for Reducing Drug Dependency and Promoting Better Asthma Control for Chronic Asthmatic Children: A Multicity Experiment.Glob Pediatr Health. 2019 Mar 19;6:2333794X19837455. doi: 10.1177/2333794X19837455. eCollection 2019. Glob Pediatr Health. 2019. PMID: 30915390 Free PMC article.
-
Psychometric properties and predictive validity of the PP-ACT.J Asthma. 2023 Jan;60(1):174-184. doi: 10.1080/02770903.2022.2036755. Epub 2022 Feb 14. J Asthma. 2023. PMID: 35094619 Free PMC article.
-
Development of Core Outcome Measures sets for paediatric and adult Severe Asthma (COMSA).Eur Respir J. 2023 Apr 3;61(4):2200606. doi: 10.1183/13993003.00606-2022. Print 2023 Apr. Eur Respir J. 2023. PMID: 36229046 Free PMC article.
-
Content validity of a newly developed observer-reported measure for pediatric asthma in children aged 2-5 years.J Patient Rep Outcomes. 2022 May 28;6(1):55. doi: 10.1186/s41687-022-00461-y. J Patient Rep Outcomes. 2022. PMID: 35633412 Free PMC article.
References
-
- Expert Panel Report 3 (EPR-3). Guidelines for the Diagnosis and Management of Asthma, Summary Report 2007. National Asthma Education and Prevention Program Expert Panel Report 3. 120(Supplement 1): S94-S138 (2007). - PubMed
-
- Cloutier M. M. et al. Asthma outcomes: Composite scores of asthma control. Standardizing Asthma Outcomes in Clinical Research: Report of the Asthma Outcomes Workshop 129(3, Supplement): S24–S33 (2012).
-
- Liu A. H. et al. Development and cross-sectional validation of the Childhood Asthma Control Test. J. Allerg. Clin. Immunol. 119, 817–825 (2007). - PubMed
-
- Gerald, J. K. et al. Measurement characteristics of the pediatric asthma health outcome measure. J. Asthma 49, 260–266 (2012). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical