Human Atg8-cardiolipin interactions in mitophagy: Specific properties of LC3B, GABARAPL2 and GABARAP
- PMID: 27764541
- PMCID: PMC5172498
- DOI: 10.1080/15548627.2016.1240856
Human Atg8-cardiolipin interactions in mitophagy: Specific properties of LC3B, GABARAPL2 and GABARAP
Abstract
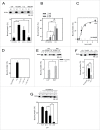
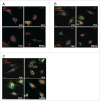
The phospholipid cardiolipin (CL) has been proposed to play a role in selective mitochondrial autophagy, or mitophagy. CL externalization to the outer mitochondrial membrane would act as a signal for the human Atg8 ortholog subfamily, MAP1LC3 (LC3). The latter would mediate both mitochondrial recognition and autophagosome formation, ultimately leading to removal of damaged mitochondria. We have applied quantitative biophysical techniques to the study of CL interaction with various Atg8 human orthologs, namely LC3B, GABARAPL2 and GABARAP. We have found that LC3B interacts preferentially with CL over other di-anionic lipids, that CL-LC3B binding occurs with positive cooperativity, and that the CL-LC3B interaction relies only partially on electrostatic forces. CL-induced increased membrane fluidity appears also as an important factor helping LC3B to bind CL. The LC3B C terminus remains exposed to the hydrophilic environment after protein binding to CL-enriched membranes. In intact U87MG human glioblastoma cells rotenone-induced autophagy leads to LC3B translocation to mitochondria and subsequent delivery of mitochondria to lysosomes. We have also observed that GABARAP, but not GABARAPL2, interacts with CL in vitro. However neither GABARAP nor GABARAPL2 were translocated to mitochondria in rotenone-treated U87MG cells. Thus the various human Atg8 orthologs might play specific roles in different autophagic processes.
Keywords: CL; GABARAP; GABARAPL2; LC3B; glioblastoma; mitophagy.
Figures







References
-
- Mizushima N, Komatsu M. Autophagy: Renovation of cells and tissues. Cell 2015; 147:728-41; http://dx.doi.org/ 10.1016/j.cell.2011.10.026 - DOI - PubMed
-
- Shibutani ST, Yoshimori T. A current perspective of autophagosome biogenesis. Cell Res 2014; 24:58-68; PMID:24296784; http://dx.doi.org/ 10.1038/cr.2013.159 - DOI - PMC - PubMed
-
- Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol 2011; 12:9-14; PMID:21179058; http://dx.doi.org/ 10.1038/nrm3028 - DOI - PMC - PubMed
-
- Ashrafi G, Schwarz TL The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differentiation 2013; 20(1):31-42; PMID:22743996; http://dx.doi.org/17166847 10.1038/cdd.2012.81 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
