Ebola vaccines in clinical trial: The promising candidates
- PMID: 27764560
- PMCID: PMC5287303
- DOI: 10.1080/21645515.2016.1225637
Ebola vaccines in clinical trial: The promising candidates
Abstract
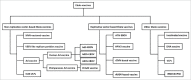
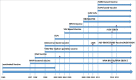
Ebola virus disease (EVD) has become a great threat to humans across the world in recent years. The 2014 Ebola epidemic in West Africa caused numerous deaths and attracted worldwide attentions. Since no specific drugs and treatments against EVD was available, vaccination was considered as the most promising and effective method of controlling this epidemic. So far, 7 vaccine candidates had been developed and evaluated through clinical trials. Among them, the recombinant vesicular stomatitis virus-based vaccine (rVSV-EBOV) is the most promising candidate, which demonstrated a significant protection against EVD in phase III clinical trial. However, several concerns were still associated with the Ebola vaccine candidates, including the safety profile in some particular populations, the immunization schedule for emergency vaccination, and the persistence of the protection. We retrospectively reviewed the current development of Ebola vaccines and discussed issues and challenges remaining to be investigated in the future.
Keywords: Ebola virus; clinical trial; efficacy; immunogenicity; safety; vaccine.
Figures

References
-
- Rimoin AW, Hotez PJ. NTDs in the heart of darkness: the Democratic Republic of Congo's unknown burden of neglected tropical diseases. PLoS Negl Trop Dis 2013; 7:e2118; PMID:23936557; http://dx.doi.org/10.1371/journal.pntd.0002118 - DOI - PMC - PubMed
-
- Towner JS, Sealy TK, Khristova ML, Albarino CG, Conlan S, Reeder SA, Quan PL, Lipkin WI, Downing R, Tappero JW, et al.. Newly discovered ebola virus associated with hemorrhagic fever outbreak in Uganda. PLoS Pathog 2008; 4:e1000212; PMID:19023410; http://dx.doi.org/10.1371/journal.ppat.1000212 - DOI - PMC - PubMed
-
- Mahanty S, Bray M. Pathogenesis of filoviral haemorrhagic fevers. Lancet Infect Dis 2004; 4:487-98; PMID:15288821; http://dx.doi.org/10.1016/S1473-3099(04)01103-X - DOI - PubMed
-
- Pourrut X, Kumulungui B, Wittmann T, Moussavou G, Delicat A, Yaba P, Nkoghe D, Gonzalez JP, Leroy EM. The natural history of Ebola virus in Africa. Microbes infect 2005; 7:1005-14; PMID:16002313; http://dx.doi.org/10.1016/j.micinf.2005.04.006 - DOI - PubMed
-
- Reed DS, Mohamadzadeh M. Status and challenges of filovirus vaccines. Vaccine 2007; 25:1923-34; PMID:17241710; http://dx.doi.org/10.1016/j.vaccine.2006.11.037 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
