Leucine Zipper Down-regulated in Cancer-1 (LDOC1) interacts with Guanine nucleotide binding protein-like 3-like (GNL3L) to modulate Nuclear Factor-kappa B (NF-κB) signaling during cell proliferation
- PMID: 27764577
- PMCID: PMC5176145
- DOI: 10.1080/15384101.2016.1242534
Leucine Zipper Down-regulated in Cancer-1 (LDOC1) interacts with Guanine nucleotide binding protein-like 3-like (GNL3L) to modulate Nuclear Factor-kappa B (NF-κB) signaling during cell proliferation
Abstract
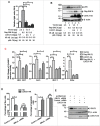
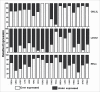
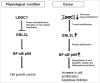
Guanine nucleotide binding protein-like 3-like (GNL3L) is an evolutionarily conserved putative nucleolar GTPase belonging to the HSR1-MMR1 family. In the present study, using protein-protein interaction assays, we show that Leucine Zipper Down-regulated in Cancer-1 (LDOC1) is a novel interacting partner of GNL3L. Furthermore, our results reveal that ectopic expression of LDOC1 destabilizes endogenous GNL3L levels and down modulates GNL3L-induced cell proliferation, in contrast, the knockdown of LDOC1 potentiates cell proliferation upon GNL3L expression. Interestingly, GNL3L upregulates NF-κB dependent transcriptional activity by modulating the expression of NF-κB subunit p65, which is reversed upon co-expression of LDOC1 with GNL3L. GNL3L also potentiates TNF-α mediated NF-κB activity. In addition, anti-apoptotic function of GNL3L is impaired upon p65 knockdown, suggesting its critical role in GNL3L mediated cell proliferation/survival. An inverse correlation of GNL3L and LDOC1 expression profiles in various tumor tissues from BioXpress database indicate their critical role in cancer. Collectively, our data provides evidence that GNL3L-LDOC1 interplay regulates cell proliferation through the modulation of NF-κB pathway during tumorigenesis.
Keywords: Apoptosis; GNL3L; LDOC1; NF-κB transcription factor; cell signaling; proliferation; protein-protein interaction.
Figures





Similar articles
-
LDOC1 inhibits proliferation and promotes apoptosis by repressing NF-κB activation in papillary thyroid carcinoma.J Exp Clin Cancer Res. 2015 Dec 4;34:146. doi: 10.1186/s13046-015-0265-z. J Exp Clin Cancer Res. 2015. PMID: 26637328 Free PMC article.
-
LDOC1 is differentially expressed in thyroid cancer and display tumor-suppressive function in papillary thyroid carcinoma.Cell Biol Int. 2020 Apr;44(4):985-997. doi: 10.1002/cbin.11295. Epub 2020 Jan 17. Cell Biol Int. 2020. PMID: 31889386
-
NF-κB upregulation through epigenetic silencing of LDOC1 drives tumor biology and specific immunophenotype in Group A ependymoma.Neuro Oncol. 2017 Oct 1;19(10):1350-1360. doi: 10.1093/neuonc/nox061. Neuro Oncol. 2017. PMID: 28510691 Free PMC article.
-
Reprogramming RelA.Cell Cycle. 2004 Jul;3(7):869-72. Epub 2004 Jul 4. Cell Cycle. 2004. PMID: 15190199 Review.
-
Specification of DNA binding activity of NF-kappaB proteins.Cold Spring Harb Perspect Biol. 2009 Oct;1(4):a000067. doi: 10.1101/cshperspect.a000067. Cold Spring Harb Perspect Biol. 2009. PMID: 20066093 Free PMC article. Review.
Cited by
-
The non-enzymatic RAS effector RASSF7 inhibits oncogenic c-Myc function.J Biol Chem. 2018 Oct 5;293(40):15691-15705. doi: 10.1074/jbc.RA118.004452. Epub 2018 Aug 23. J Biol Chem. 2018. PMID: 30139745 Free PMC article.
-
Novel STAT3 Inhibitor LDOC1 Targets Phospho-JAK2 for Degradation by Interacting with LNX1 and Regulates the Aggressiveness of Lung Cancer.Cancers (Basel). 2019 Jan 9;11(1):63. doi: 10.3390/cancers11010063. Cancers (Basel). 2019. PMID: 30634502 Free PMC article.
-
Bile Ductal Transcriptome Identifies Key Pathways and Hub Genes in Clonorchis sinensis-Infected Sprague-Dawley Rats.Korean J Parasitol. 2020 Oct;58(5):513-525. doi: 10.3347/kjp.2020.58.5.513. Epub 2020 Oct 22. Korean J Parasitol. 2020. PMID: 33202503 Free PMC article.
-
GNL3L exhibits pro-tumor activities via NF-κB pathway as a poor prognostic factor in acute myeloid leukemia.J Cancer. 2024 May 30;15(13):4072-4080. doi: 10.7150/jca.95339. eCollection 2024. J Cancer. 2024. PMID: 38947394 Free PMC article.
-
Searching for the 'X' factor: investigating the genetics of primary ovarian insufficiency.J Ovarian Res. 2024 Nov 28;17(1):238. doi: 10.1186/s13048-024-01555-5. J Ovarian Res. 2024. PMID: 39609914 Free PMC article. Review.
References
-
- Subba Rao MRK, Kumari G, Balasundaram D, Sankaranarayanan R, Mahalingam SA. Novel lysine-rich domain and gtp binding motifs regulate the nucleolar retention of human guanine nucleotide binding protein, GNL3L. J Mol Biol 2006; 364:637-54; PMID:17034816; http://dx.doi.org/10.1016/j.jmb.2006.09.007 - DOI - PubMed
-
- Meng L, Zhu Q, Tsai R. Nucleolar trafficking of nucleostemin family proteins: common versus protein-specific mechanisms. Mol Cell Biol 2007; 27:8670-82; PMID:17923687; http://dx.doi.org/10.1128/MCB.00635-07 - DOI - PMC - PubMed
-
- Meng L, Hsu JK, Tsai RYL. GNL3L depletion destabilizes MDM2 and induces p53-dependent G2/M arrest. Oncogene 2010; 30:1716-26; PMID:21132010; http://dx.doi.org/10.1038/onc.2010.550 - DOI - PMC - PubMed
-
- Yasumoto H, Meng L, Lin T, Zhu Q, Tsai R. GNL3L inhibits activity of estrogen-related receptor γ by competing for co-activator binding. J Cell Sci 2007; 120:2532-43; PMID:17623774; http://dx.doi.org/10.1242/jcs.009878 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
