Rapid Sensory Adaptation Redux: A Circuit Perspective
- PMID: 27764664
- PMCID: PMC5076890
- DOI: 10.1016/j.neuron.2016.09.046
Rapid Sensory Adaptation Redux: A Circuit Perspective
Abstract
Adaptation is fundamental to life. All organisms adapt over timescales that span from evolution to generations and lifetimes to moment-by-moment interactions. The nervous system is particularly adept at rapidly adapting to change, and this in fact may be one of its fundamental principles of organization and function. Rapid forms of sensory adaptation have been well documented across all sensory modalities in a wide range of organisms, yet we do not have a comprehensive understanding of the adaptive cellular mechanisms that ultimately give rise to the corresponding percepts, due in part to the complexity of the circuitry. In this Perspective, we aim to build links between adaptation at multiple scales of neural circuitry by investigating the differential adaptation across brain regions and sub-regions and across specific cell types, for which the explosion of modern tools has just begun to enable. This investigation points to a set of challenges for the field to link functional observations to adaptive properties of the neural circuit that ultimately underlie percepts.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures

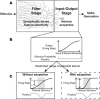
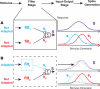

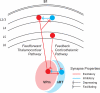
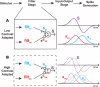
References
-
- Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic Depression and Cortical Gain Control. Science (80−. ) 1997;275:221–224. - PubMed
-
- Abeles M. Role of the cortical neuron: integrator or coincidence detector? Isr. J. Med. Sci. 1982;18:83–92. - PubMed
-
- Ahissar E, Sosnik R, Haidarliu S. Transformation from temporal to rate coding in a somatosensory thalamocortical pathway. Nature. 2000;406:302–306. - PubMed
-
- Allman J, Miezin F, McGuinness E. Stimulus specific responses from beyond the classical receptive field : Neurophysiological Mechanisms for Local-Global Comparisons in Visual Neurons. Annu. Rev. Neurosci. 1985;8:407–430. - PubMed
-
- Anderson J, Lampl I, Reichova I, Carandini M, Ferster D. Stimulus dependence of two-state fluctuations of membrane potential in cat visual cortex. Nat. Neurosci. 2000;3:617–621. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

