Impact of tumour RAS/BRAF status in a first-line study of panitumumab + FOLFIRI in patients with metastatic colorectal cancer
- PMID: 27764839
- PMCID: PMC5104899
- DOI: 10.1038/bjc.2016.343
Impact of tumour RAS/BRAF status in a first-line study of panitumumab + FOLFIRI in patients with metastatic colorectal cancer
Abstract
Background: To investigate tumour biomarker status and efficacy of first-line panitumumab+FOLFIRI for metastatic colorectal carcinoma (mCRC).
Methods: 154 patients received first-line panitumumab + FOLFIRI every 14 days. Primary end point was objective response rate (ORR). Data were analysed by tumour RAS (KRAS/NRAS) and BRAF status, and baseline amphiregulin (AREG) expression.
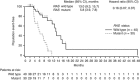
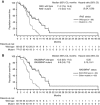
Results: Objective responses occurred more frequently in RAS wild type (WT) (59%) vs RAS mutant (MT) (41%) mCRC and in RAS WT/BRAF WT (68%) vs RAS or BRAF MT (37%) disease. Median response duration was longer in RAS WT (13.0 months) vs RAS MT (5.8 months) (hazard ratio (HR): 0.16). Median progression-free survival was longer in RAS WT vs MT (11.2 vs 7.3 months; HR, 0.37) and was also longer in RAS WT/BRAF WT vs RAS or BRAF MT (13.2 vs 6.9 months; HR, 0.25). Incidence of adverse events was similar regardless of RAS/BRAF status, and no new safety signals were noted. Among patients with RAS WT tumours, ORR was 67% with high AREG expression and 38% with low AREG expression.
Conclusions: First-line panitumumab+FOLFIRI was associated with favourable efficacy in patients with RAS WT and RAS WT/BRAF WT vs MT mCRC tumours and was well tolerated.
Conflict of interest statement
MK is an advisor for, and has received honoraria from, Amgen Ltd. R-DH has received honoraria from Amgen Ltd. LM is an advisor for, and has received honoraria and research funding from, Amgen. HL has acted as a consultant/advisor for Amgen Ltd & Roche Ltd. RG has received research support from Amgen. JT has received honoraria and research funding from Amgen Ltd. KSO is a former employee of Amgen Inc. MB, BT, YZ, GD are employees of Amgen Inc. C-HK has acted as a consultant/advisor to Amgen Ltd, Merck KG Darmstadt & Roche Ltd. EF declares no conflict of interest.
Figures
References
-
- Abad A, Massuti B, Gravalos C, Escudero P, Guillen C, Manzano JL, Gomez MA, Safont MJ, Plazas JG, Sastre J, Pericay C, Dueñas R, López-López C, Losa F, Ayerbes MV, González E, Yuste A, Carrato A, Aranda E (2014) Phase II trial of panitumumab plus FOLFOX4 or FOLFIRI in subjects with KRAS wild-type colorectal cancer and liver-limited disease: the PLANET study. J Clin Oncol 32(5 Suppl): Abstract 3560.
-
- Bokemeyer C, Bondarenko I, Hartmann JT, de Braud F, Schuch G, Zubel A, Celik I, Schlichting M, Koralewski P (2011) Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol 22: 1535–1546. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous