Ridaforolimus (MK-8669) synergizes with Dalotuzumab (MK-0646) in hormone-sensitive breast cancer
- PMID: 27765027
- PMCID: PMC5073873
- DOI: 10.1186/s12885-016-2847-3
Ridaforolimus (MK-8669) synergizes with Dalotuzumab (MK-0646) in hormone-sensitive breast cancer
Abstract
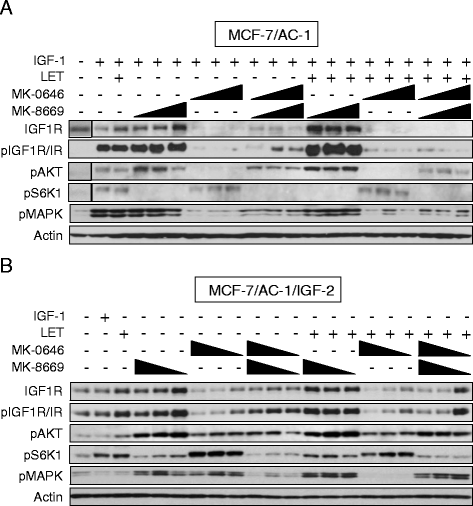
Background: Mammalian target of rapamycin (mTOR) represents a key downstream intermediate for a myriad of oncogenic receptor tyrosine kinases. In the case of the insulin-like growth factor (IGF) pathway, the mTOR complex (mTORC1) mediates IGF-1 receptor (IGF-1R)-induced estrogen receptor alpha (ERα) phosphorylation/activation and leads to increased proliferation and growth in breast cancer cells. As a result, the prevalence of mTOR inhibitors combined with hormonal therapy has increased in recent years. Conversely, activated mTORC1 provides negative feedback regulation of IGF signaling via insulin receptor substrate (IRS)-1/2 serine phosphorylation and subsequent proteasomal degradation. Thus, the IGF pathway may provide escape (e.g. de novo or acquired resistance) from mTORC1 inhibitors. It is therefore plausible that combined inhibition of mTORC1 and IGF-1R for select subsets of ER-positive breast cancer patients presents as a viable therapeutic option.
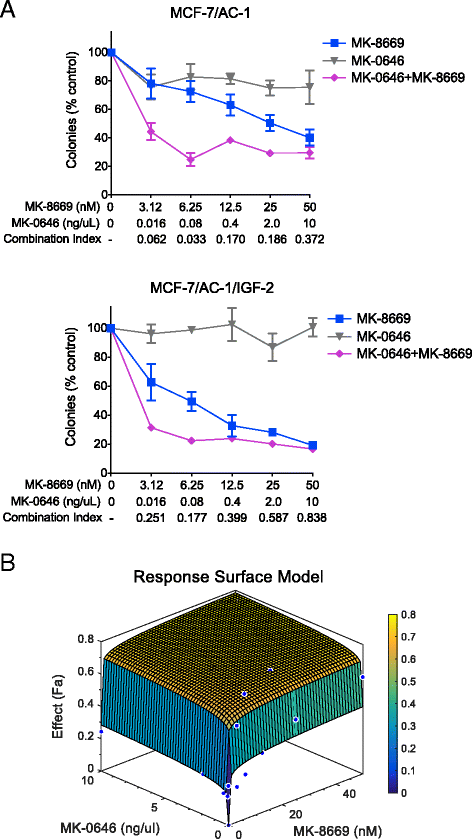
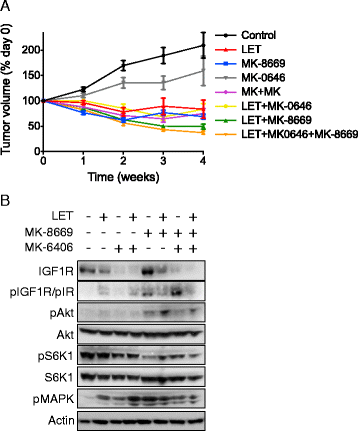
Methods: Using hormone-sensitive breast cancer cells stably transfected with the aromatase gene (MCF-7/AC-1), works presented herein describe the in vitro and in vivo antitumor efficacy of the following compounds: dalotuzumab (DALO; "MK-0646"; anti-IGF-1R antibody), ridaforolimus (RIDA; "MK-8669"; mTORC1 small molecule inhibitor) and letrozole ("LET", aromatase inhibitor).
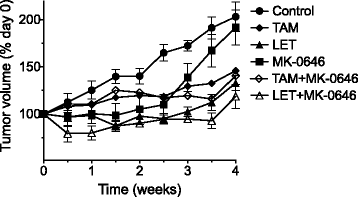
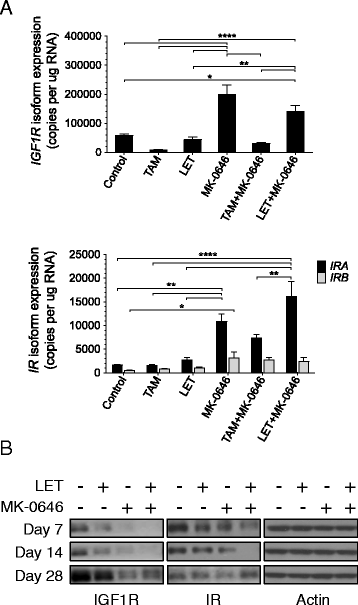
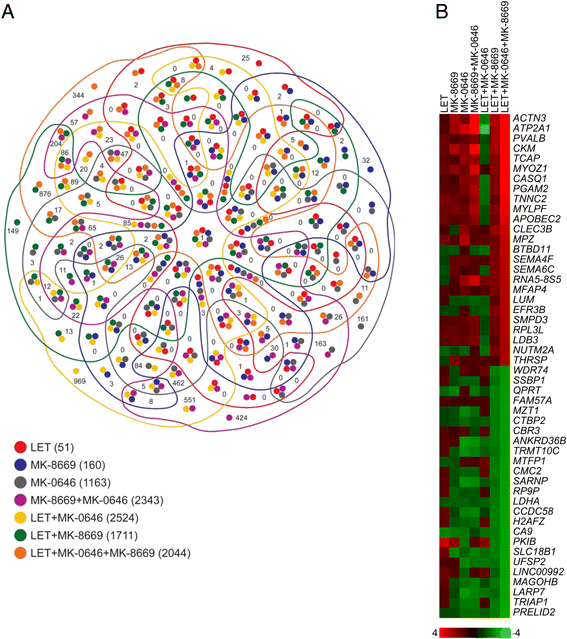
Results: With the exception of MK-0646, all single agent and combination treatment arms effectively inhibited xenograft tumor growth, albeit to varying degrees. Correlative tissue analyses revealed MK-0646 alone and in combination with LET induced insulin receptor alpha A (InsR-A) isoform upregulation (both mRNA and protein expression), thereby further supporting a triple therapy approach.
Conclusion: These data provide preclinical rationalization towards the combined triple therapy of LET plus MK-0646 plus MK-8669 as an efficacious anti-tumor strategy for ER-positive breast tumors.
Keywords: Aromatase inhibitors/therapeutic use; Breast neoplasms/drug therapy; Disease models, Animal; Drug resistance, Neoplasm; Receptor, IGF type 1; Receptor, Insulin; mTOR inhibitor.
Figures
References
-
- Di Cosimo S, Sathyanarayanan S, Bendell JC, Cervantes A, Stein MN, Brana I, et al. Combination of the mTOR Inhibitor Ridaforolimus and the Anti-IGF1R Monoclonal Antibody Dalotuzumab: Preclinical Characterization and Phase I Clinical Trial. Clin. Cancer Res. 2015;21:49–59. doi: 10.1158/1078-0432.CCR-14-0940. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

