Microtubule Motors Drive Hedgehog Signaling in Primary Cilia
- PMID: 27765513
- PMCID: PMC5258846
- DOI: 10.1016/j.tcb.2016.09.010
Microtubule Motors Drive Hedgehog Signaling in Primary Cilia
Abstract
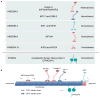
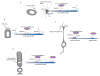
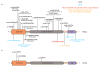
The mammalian Hedgehog (Hh) signaling pathway is required for development and for maintenance of adult stem cells, and overactivation of the pathway can cause tumorigenesis. All responses to Hh family ligands in mammals require the primary cilium, an ancient microtubule-based organelle that extends from the cell surface. Genetic studies in mice and humans have defined specific functions for cilium-associated microtubule motor proteins: they act in the construction and disassembly of the primary cilium, they control ciliary length and stability, and some have direct roles in mammalian Hh signal transduction. These studies highlight how integrated genetic and cell biological studies can define the molecular mechanisms that underlie cilium-associated health and disease.
Keywords: Hedgehog pathway; ciliopathies; dynein; kinesin; microtubules; primary cilia.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures

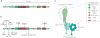
References
-
- Scholey JM, Anderson KV. Intraflagellar transport and cilium-based signaling. Cell. 2006;125:439–442. - PubMed
-
- Huangfu D, et al. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. - PubMed
-
- Ingham PW, et al. Mechanisms and functions of Hedgehog signalling across the metazoa. Nat Rev Genet. 2011;12:393–406. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

