Cisplatin induces mitochondrial deficits in Drosophila larval segmental nerve
- PMID: 27765583
- PMCID: PMC5138083
- DOI: 10.1016/j.nbd.2016.10.003
Cisplatin induces mitochondrial deficits in Drosophila larval segmental nerve
Abstract
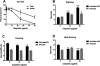
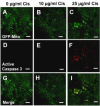
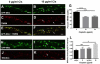
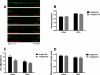
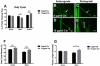
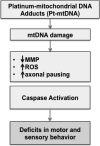
Cisplatin is an effective chemotherapy drug that induces peripheral neuropathy in cancer patients. In rodent dorsal root ganglion neurons, cisplatin binds nuclear and mitochondrial DNA (mtDNA) inducing DNA damage and apoptosis. Platinum-mtDNA adducts inhibit mtDNA replication and transcription leading to mitochondrial degradation. Cisplatin also induces climbing deficiencies associated with neuronal apoptosis in adult Drosophila melanogaster. Here we used Drosophila larvae that express green fluorescent protein in the mitochondria of motor neurons to observe the effects of cisplatin on mitochondrial dynamics and function. Larvae treated with 10μg/ml cisplatin had normal survival with deficiencies in righting and heat sensing behavior. Behavior was abrogated by, the pan caspase inhibitor, p35. However, active caspase 3 was not detected by immunostaining. There was a 27% decrease in mitochondrial membrane potential and a 42% increase in reactive oxygen species (ROS) in mitochondria along the axon. Examination of mitochondrial axonal trafficking showed no changes in velocity, flux or mitochondrial length. However, cisplatin treatment resulted in a greater number of stationary organelles caused by extended pausing during axonal motility. These results demonstrate that cisplatin induces behavior deficiencies in Drosophila larvae, decreased mitochondrial activity, increased ROS production and mitochondrial pausing without killing the larvae. Thus, we identified particular aspects of mitochondrial dynamics and function that are affected in cisplatin-induced peripheral neuropathy and may represent key therapeutic targets.
Keywords: Apoptosis; Axonal trafficking; Cisplatin; Drosophila; Membrane potential; Mitochondria; Motor neuron.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Compartmentalized Regulation of Parkin-Mediated Mitochondrial Quality Control in the Drosophila Nervous System In Vivo.J Neurosci. 2016 Jul 13;36(28):7375-91. doi: 10.1523/JNEUROSCI.0633-16.2016. J Neurosci. 2016. PMID: 27413149 Free PMC article.
-
ROS regulation of axonal mitochondrial transport is mediated by Ca2+ and JNK in Drosophila.PLoS One. 2017 May 18;12(5):e0178105. doi: 10.1371/journal.pone.0178105. eCollection 2017. PLoS One. 2017. PMID: 28542430 Free PMC article.
-
Drosophila melanogaster: a new model to study cisplatin-induced neurotoxicity.Neurobiol Dis. 2011 Aug;43(2):330-7. doi: 10.1016/j.nbd.2011.03.022. Epub 2011 Apr 15. Neurobiol Dis. 2011. PMID: 21514385 Free PMC article.
-
Apoptosis in Drosophila: which role for mitochondria?Apoptosis. 2016 Mar;21(3):239-51. doi: 10.1007/s10495-015-1209-y. Apoptosis. 2016. PMID: 26679112 Review.
-
Lack of correlation between mitochondrial reactive oxygen species production and life span in Drosophila.Ann N Y Acad Sci. 2004 Jun;1019:388-91. doi: 10.1196/annals.1297.069. Ann N Y Acad Sci. 2004. PMID: 15247051 Review.
Cited by
-
Genetic Reduction of Mitochondria Complex I Subunits is Protective against Cisplatin-Induced Neurotoxicity in Drosophila.J Neurosci. 2022 Feb 2;42(5):922-937. doi: 10.1523/JNEUROSCI.1479-20.2021. Epub 2021 Dec 10. J Neurosci. 2022. PMID: 34893548 Free PMC article.
-
Insights into platinum-induced peripheral neuropathy-current perspective.Neural Regen Res. 2020 Sep;15(9):1623-1630. doi: 10.4103/1673-5374.276321. Neural Regen Res. 2020. PMID: 32209761 Free PMC article. Review.
-
Platinum-induced neurotoxicity: A review of possible mechanisms.World J Clin Oncol. 2017 Aug 10;8(4):329-335. doi: 10.5306/wjco.v8.i4.329. World J Clin Oncol. 2017. PMID: 28848699 Free PMC article. Review.
-
Oxaliplatin-induced peripheral neuropathy: clinical features, mechanisms, prevention and treatment.J Neurol. 2021 Sep;268(9):3269-3282. doi: 10.1007/s00415-020-09942-w. Epub 2020 May 30. J Neurol. 2021. PMID: 32474658 Review.
-
Platinum-Induced Peripheral Neuropathy (PIPN): ROS-Related Mechanism, Therapeutic Agents, and Nanosystems.Front Mol Biosci. 2021 Nov 24;8:770808. doi: 10.3389/fmolb.2021.770808. eCollection 2021. Front Mol Biosci. 2021. PMID: 34901160 Free PMC article. Review.
References
-
- Carozzi VA, et al. Chemotherapy-induced peripheral neuropathy: What do we know about mechanisms? Neurosci Lett. 2015;596:90–107. - PubMed
-
- Cavaletti G, Marmiroli P. Chemotherapy-induced peripheral neurotoxicity. Nature reviews. Neurology. 2010;6:657–66. - PubMed
-
- Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

