Staphylococcus aureus Induces Increased Serine Protease Activity in Keratinocytes
- PMID: 27765722
- PMCID: PMC5258850
- DOI: 10.1016/j.jid.2016.10.008
Staphylococcus aureus Induces Increased Serine Protease Activity in Keratinocytes
Abstract
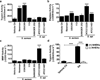
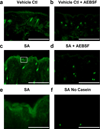
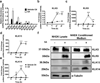
Bacteria that reside on the skin can influence the behavior of the cutaneous immune system, but the mechanisms responsible for these effects are incompletely understood. Colonization of the skin by Staphylococcus aureus (S. aureus) is increased in atopic dermatitis and can result in increased severity of the disease. In this study, we show that S. aureus stimulates human keratinocytes to increase their endogenous protease activity, including specific increases in trypsin activity. This increased protease activity coincided with increased expression of mRNA for kallikreins (KLKs), with KLK6, 13, and 14 showing the greatest induction after exposure to S. aureus. Suppression of mRNA for these KLKs in keratinocytes by targeted small interfering RNA silencing before S. aureus exposure blocked the increase in protease activity. Keratinocytes exposed to S. aureus showed enhanced degradation of desmoglein-1 and filaggrin, whereas small interfering RNA for KLK6, KLK13, and KLK14 partially blocked this degradation. These data illustrate how S. aureus directly influences the skin barrier integrity by stimulating endogenous proteolytic activity and defines a previously unknown mechanism by which S. aureus may influence skin diseases.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Belkaid Y, Segre JA. Dialogue between skin microbiota and immunity. Science. 2014;346(6212):7. - PubMed
-
- Borgono CA, Michael IP, Komatsu N, Jayakumar A, Kapadia R, Clayman GL, et al. A potential role for multiple tissue kallikrein serine proteases in epidermal desquamation. J Biol Chem. 2007;282(6):3640–3652. - PubMed
-
- Caubet C, Jonca N, Brattsand M, Guerrin M, Bernard D, Schmidt R, et al. Degradation of corneodesmosome proteins by two serine proteases of the kallikrein family, SCTE/KLK5/hK5 and SCCE/KLK7/hK7. J Invest Dermatol. 2004;122(5):1235–1244. - PubMed
-
- Chavanas S, Bodemer C, Rochat A, Hamel-Teillac D, Ali M, Irvine AD, et al. Mutations in SPINK5, encoding a serine protease inhibitor, cause Netherton syndrome. Nature Genetics. 2000;25(2):2. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

