Current animal models of hemophilia: the state of the art
- PMID: 27766048
- PMCID: PMC5056469
- DOI: 10.1186/s12959-016-0106-0
Current animal models of hemophilia: the state of the art
Abstract
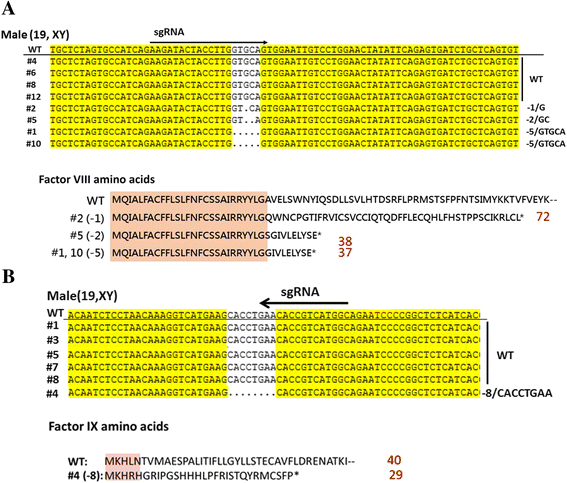
Hemophilia is the most well-known hereditary bleeding disorder, with an incidence of one in every 5000 to 30,000 males worldwide. The disease is treated by infusion of protein products on demand and as prophylaxis. Although these therapies have been very successful, some challenging and unresolved tasks remain, such as reducing bleeding rates, presence of target joints and/or established joint damage, eliminating the development of inhibitors, and increasing the success rate of immune-tolerance induction (ITI). Many preclinical trials are carried out on animal models for hemophilia generated by the hemophilia research community, which in turn enable prospective clinical trials aiming to tackle these challenges. Suitable animal models are needed for greater advances in treating hemophilia, such as the development of better models for evaluation of the efficacy and safety of long-acting products, more powerful gene therapy vectors than are currently available, and successful ITI strategies. Mice, dogs, and pigs are the most commonly used animal models for hemophilia. With the advent of the nuclease method for genome editing, namely the CRISPR/Cas9 system, it is now possible to create animal models for hemophilia other than mice in a short period of time. This review presents currently available animal models for hemophilia, and discusses the importance of animal models for the development of better treatment options for hemophilia.
Keywords: Animal models; CRISPR/Cas9; Genetically-engineered; Hemophilia.
Figures
Similar articles
-
Targeted genome engineering in human induced pluripotent stem cells from patients with hemophilia B using the CRISPR-Cas9 system.Stem Cell Res Ther. 2018 Apr 6;9(1):92. doi: 10.1186/s13287-018-0839-8. Stem Cell Res Ther. 2018. PMID: 29625575 Free PMC article.
-
Immune tolerance induction in patients with severe hemophilia with inhibitors: expert panel views and recommendations for clinical practice.Eur J Haematol. 2012 May;88(5):371-9. doi: 10.1111/j.1600-0609.2012.01754.x. Epub 2012 Feb 7. Eur J Haematol. 2012. PMID: 22260405 Review.
-
Review of immune tolerance induction in hemophilia A.Blood Rev. 2018 Jul;32(4):326-338. doi: 10.1016/j.blre.2018.02.003. Epub 2018 Feb 15. Blood Rev. 2018. PMID: 29482894 Review.
-
Defining the impact of hemophilia: the Academic Achievement in Children with Hemophilia Study.Pediatrics. 2001 Dec;108(6):E105. doi: 10.1542/peds.108.6.e105. Pediatrics. 2001. PMID: 11731632
-
Inhibitors in Hemophilia: Treatment Challenges and Novel Options.Semin Thromb Hemost. 2018 Sep;44(6):544-550. doi: 10.1055/s-0037-1612626. Epub 2017 Dec 12. Semin Thromb Hemost. 2018. PMID: 29232720 Review.
Cited by
-
Assessment of the percentage of full recombinant adeno-associated virus particles in a gene therapy drug using CryoTEM.PLoS One. 2022 Jun 3;17(6):e0269139. doi: 10.1371/journal.pone.0269139. eCollection 2022. PLoS One. 2022. PMID: 35657790 Free PMC article.
-
CRISPR/Cas9 mediated generation of zebrafish f9a mutant as a model for hemophilia B.Blood Coagul Fibrinolysis. 2025 Apr 1;36(3):90-98. doi: 10.1097/MBC.0000000000001355. Epub 2025 Mar 19. Blood Coagul Fibrinolysis. 2025. PMID: 40127118
-
A Foundational Study for Normal F8-Containing Mouse Models for the miRNA Regulation of Hemophilia A: Identification and Analysis of Mouse miRNAs that Downregulate the Murine F8 Gene.Int J Mol Sci. 2020 Aug 6;21(16):5621. doi: 10.3390/ijms21165621. Int J Mol Sci. 2020. PMID: 32781510 Free PMC article.
-
Escape or Fight: Inhibitors in Hemophilia A.Front Immunol. 2020 Mar 24;11:476. doi: 10.3389/fimmu.2020.00476. eCollection 2020. Front Immunol. 2020. PMID: 32265927 Free PMC article. Review.
-
Gene Therapy Approaches for the Treatment of Hemophilia B.Int J Mol Sci. 2023 Jun 28;24(13):10766. doi: 10.3390/ijms241310766. Int J Mol Sci. 2023. PMID: 37445943 Free PMC article. Review.
References
-
- Giles AR, Tinlin S, Greenwood R. A canine model of hemophilic (factor VIII:C deficiency) bleeding. Blood. 1982;60:727–30. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

