Hemophilia A gene therapy via intraosseous delivery of factor VIII-lentiviral vectors
- PMID: 27766066
- PMCID: PMC5056462
- DOI: 10.1186/s12959-016-0105-1
Hemophilia A gene therapy via intraosseous delivery of factor VIII-lentiviral vectors
Abstract
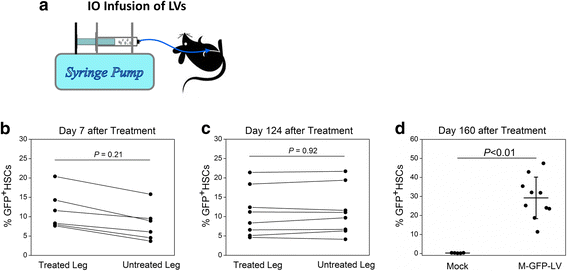
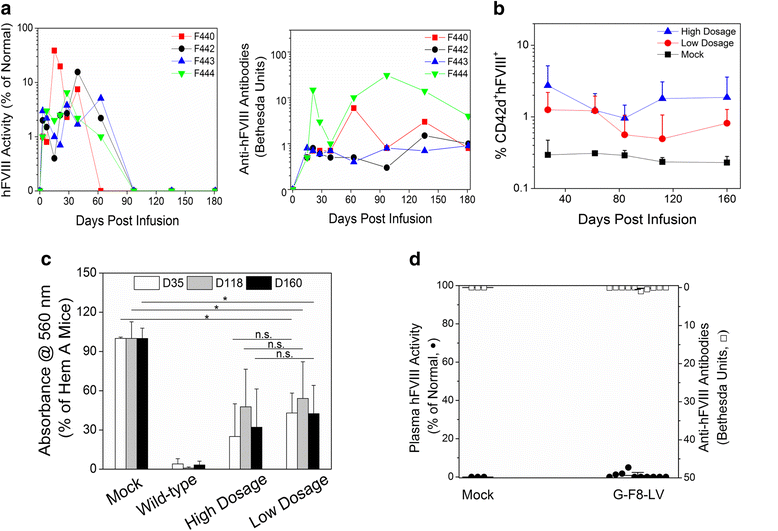
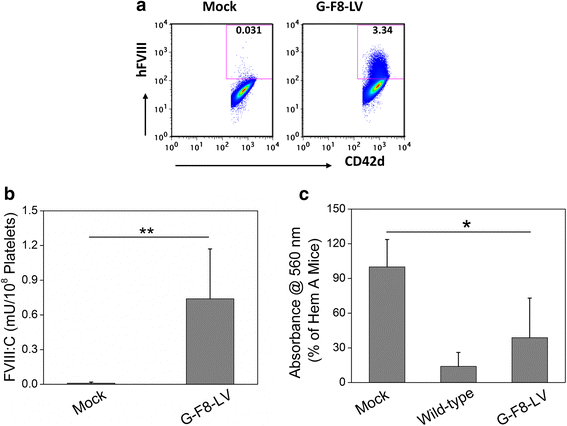
Current treatment of hemophilia A (HemA) patients with repeated infusions of factor VIII (FVIII; abbreviated as F8 in constructs) is costly, inconvenient, and incompletely effective. In addition, approximately 25 % of treated patients develop anti-factor VIII immune responses. Gene therapy that can achieve long-term phenotypic correction without the complication of anti-factor VIII antibody formation is highly desired. Lentiviral vector (LV)-mediated gene transfer into hematopoietic stem cells (HSCs) results in stable integration of FVIII gene into the host genome, leading to persistent therapeutic effect. However, ex vivo HSC gene therapy requires pre-conditioning which is highly undesirable for hemophilia patients. The recently developed novel methodology of direct intraosseous (IO) delivery of LVs can efficiently transduce bone marrow cells, generating high levels of transgene expression in HSCs. IO delivery of E-F8-LV utilizing a ubiquitous EF1α promoter generated initially therapeutic levels of FVIII, however, robust anti-FVIII antibody responses ensued neutralized functional FVIII activity in the circulation. In contrast, a single IO delivery of G-FVIII-LV utilizing a megakaryocytic-specific GP1bα promoter achieved platelet-specific FVIII expression, leading to persistent, partial correction of HemA in treated animals. Most interestingly, comparable therapeutic benefit with G-F8-LV was obtained in HemA mice with pre-existing anti-FVIII inhibitors. Platelets is an ideal IO delivery vehicle since FVIII stored in α-granules of platelets is protected from high-titer anti-FVIII antibodies; and that even relatively small numbers of activated platelets that locally excrete FVIII may be sufficient to promote efficient clot formation during bleeding. Additionally, combination of pharmacological agents improved transduction of LVs and persistence of transduced cells and transgene expression. Overall, a single IO infusion of G-F8-LV can generate long-term stable expression of hFVIII in platelets and correct hemophilia phenotype for long term. This approach has high potential to permanently treat FVIII deficiency with and without pre-existing anti-FVIII antibodies.
Keywords: Anti-FVIII inhibitory antibodies; Factor VIII; Gene therapy; Hemophilia A; Intraosseous delivery; Lentiviral vectors; Megakaryocyte-specific gene expression; Stem cell gene therapy.
Figures
Similar articles
-
Intraosseous delivery of lentiviral vectors targeting factor VIII expression in platelets corrects murine hemophilia A.Mol Ther. 2015 Apr;23(4):617-26. doi: 10.1038/mt.2015.20. Epub 2015 Feb 6. Mol Ther. 2015. PMID: 25655313 Free PMC article.
-
Enhancing therapeutic efficacy of in vivo platelet-targeted gene therapy in hemophilia A mice.Blood Adv. 2020 Nov 24;4(22):5722-5734. doi: 10.1182/bloodadvances.2020002479. Blood Adv. 2020. PMID: 33216891 Free PMC article.
-
Intraosseous delivery of platelet-targeted factor VIII lentiviral vector in humanized NBSGW mice.Blood Adv. 2022 Oct 11;6(19):5556-5569. doi: 10.1182/bloodadvances.2022008079. Blood Adv. 2022. PMID: 35849710 Free PMC article.
-
The Immune Response to the fVIII Gene Therapy in Preclinical Models.Front Immunol. 2020 Apr 15;11:494. doi: 10.3389/fimmu.2020.00494. eCollection 2020. Front Immunol. 2020. PMID: 32351497 Free PMC article. Review.
-
Development of improved factor VIII molecules and new gene transfer approaches for hemophilia A.Curr Gene Ther. 2003 Feb;3(1):27-41. doi: 10.2174/1566523033347417. Curr Gene Ther. 2003. PMID: 12553533 Review.
Cited by
-
Projection Stereolithographic Fabrication of BMP-2 Gene-activated Matrix for Bone Tissue Engineering.Sci Rep. 2017 Sep 12;7(1):11327. doi: 10.1038/s41598-017-11051-0. Sci Rep. 2017. PMID: 28900122 Free PMC article.
-
Platelet-Targeted FVIII Gene Therapy Restores Hemostasis and Induces Immune Tolerance for Hemophilia A.Front Immunol. 2020 Jun 12;11:964. doi: 10.3389/fimmu.2020.00964. eCollection 2020. Front Immunol. 2020. PMID: 32595633 Free PMC article. Review.
-
Hemophilia a patients with inhibitors: Mechanistic insights and novel therapeutic implications.Front Immunol. 2022 Dec 8;13:1019275. doi: 10.3389/fimmu.2022.1019275. eCollection 2022. Front Immunol. 2022. PMID: 36569839 Free PMC article. Review.
-
Emerging Therapeutic Modalities against COVID-19.Pharmaceuticals (Basel). 2020 Aug 8;13(8):188. doi: 10.3390/ph13080188. Pharmaceuticals (Basel). 2020. PMID: 32784499 Free PMC article. Review.
-
Defining the Optimal FVIII Transgene for Placental Cell-Based Gene Therapy to Treat Hemophilia A.Mol Ther Methods Clin Dev. 2020 Mar 14;17:465-477. doi: 10.1016/j.omtm.2020.03.001. eCollection 2020 Jun 12. Mol Ther Methods Clin Dev. 2020. PMID: 32258210 Free PMC article.
References
-
- White GC., 2nd Gene therapy in hemophilia: clinical trials update. Thromb Haemost. 2001;86:172–7. - PubMed
-
- Doering CB, Gangadharan B, Dukart HZ, Spencer HT. Hematopoietic stem cells encoding porcine factor VIII induce pro-coagulant activity in hemophilia A mice with pre-existing factor VIII immunity. Mol Ther. 2007;15:1093–9. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

