Impaired Levels of Gangliosides in the Corpus Callosum of Huntington Disease Animal Models
- PMID: 27766070
- PMCID: PMC5052274
- DOI: 10.3389/fnins.2016.00457
Impaired Levels of Gangliosides in the Corpus Callosum of Huntington Disease Animal Models
Abstract
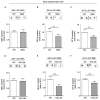
Huntington Disease (HD) is a genetic neurodegenerative disorder characterized by broad types of cellular and molecular dysfunctions that may affect both neuronal and non-neuronal cell populations. Among all the molecular mechanisms underlying the complex pathogenesis of the disease, alteration of sphingolipids has been identified as one of the most important determinants in the last years. In the present study, besides the purpose of further confirming the evidence of perturbed metabolism of gangliosides GM1, GD1a, and GT1b the most abundant cerebral glycosphingolipids, in the striatal and cortical tissues of HD transgenic mice, we aimed to test the hypothesis that abnormal levels of these lipids may be found also in the corpus callosum white matter, a ganglioside-enriched brain region described being dysfunctional early in the disease. Semi-quantitative analysis of GM1, GD1a, and GT1b content indicated that ganglioside metabolism is a common feature in two different HD animal models (YAC128 and R6/2 mice) and importantly, demonstrated that levels of these gangliosides were significantly reduced in the corpus callosum white matter of both models starting from the early stages of the disease. Besides corroborating the evidence of aberrant ganglioside metabolism in HD, here, we found out for the first time, that ganglioside dysfunction is an early event in HD models and it may potentially represent a critical molecular change influencing the pathogenesis of the disease.
Keywords: Corpus Callosum White Matter (CC-WM); GD1a; GM1; GT1b; Gangliosides; HD.
Figures




References
-
- Bohanna I., Georgiou-Karistianis N., Sritharan A., Asadi H., Johnston L., Churchyard A., et al. (2011). Diffusion tensor imaging in Huntington's disease reveals distinct patterns of white matter degeneration associated with motor and cognitive deficits. Brain Imaging Behav. 5, 171–180. 10.1007/s11682-011-9121-8 - DOI - PubMed
-
- Ciarmiello A., Cannella M., Lastoria S., Simonelli M., Frati L., Rubinsztein D. C., et al. (2006). Brain white-matter volume loss and glucose hypometabolism precede the clinical symptoms of Huntington's disease. J. Nucl. Med. 47, 215–222. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

