Spatial and compositional variation in the fungal communities of organic and conventionally grown apple fruit at the consumer point-of-purchase
- PMID: 27766161
- PMCID: PMC5051542
- DOI: 10.1038/hortres.2016.47
Spatial and compositional variation in the fungal communities of organic and conventionally grown apple fruit at the consumer point-of-purchase
Abstract
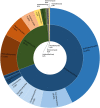
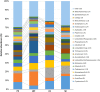
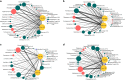
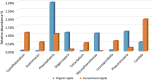
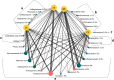
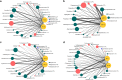
The fungal diversity in harvested apples from organic or conventional management practices was analyzed in different fruit locations (stem end, calyx end, peel, and wounded flesh) shortly after fruit purchase (T1) and after 2 weeks of storage (T5). A total of 5,760,162 high-quality fungal sequences were recovered and assigned to 8,504 Operational Taxonomic Units. Members of the phylum Ascomycota were dominant in all samples and accounted for 91.6% of the total number of detected sequences. This was followed by Basidiomycota (8%), Chytridiomycota (0.1%), and unidentified fungi (0.3%). Alpha and beta diversity analyses revealed the presence of significantly different fungal populations in the investigated fruit parts. Among detected fungi, the genus Penicillium prevailed in the peel and in the wounded flesh while Alternaria spp. prevailed in the calyx and stem end samples that included apple core tissues. Several taxonomic units that appear to be closely related to pathogenic fungi associated with secondary human infections were present in peel and wounds. Moreover, significantly different populations were revealed in organic and conventional apples and this result was consistent in all investigated fruit parts (calyx end, peel, stem end, and wounded flesh). Several unique taxa were exclusively detected in organic apples suggesting that management practices may have been a contributing factor in determining the taxa present. In contrast, little differences were revealed in the two assessment times (T1 and T5). Results of the present study represent an advancement of the current knowledge on the fungal microbiota in collected fruit tissues of apple.
Figures


References
-
- Wisniewski M, Droby S, Norelli J, Liu J, Schena L. Alternative management technologies for postharvest disease control: the journey from simplicity to complexity. Postharvest Biol Technol 2016; 122: 3–10.
-
- Droby S, Wisniewski M, Teixidó N, Spadaro D, Jijakli MH. The science, development, and commercialization of postharvest biocontrol products. Postharvest Biol Technol 2016; 122: 22–29.
-
- Busby PE, Ridout M, Newcombe G. Fungal endophytes: modifiers of plant disease. Plant Mol Biol 2016; 90: 645–655. - PubMed
-
- Mari M, Di Francesco A, Bertolini P. Control of fruit postharvest diseases: old issues and innovative approaches. Stewart Postharvest Review 2014; 10: 1–4.
-
- Peck GM, Andrews PK, Reganold JP, Fellman JK. Apple orchard productivity and fruit quality under organic, conventional, and integrated management. Hortic Sci 2006; 41: 99–107.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

