ABC Transporter Subfamily D: Distinct Differences in Behavior between ABCD1-3 and ABCD4 in Subcellular Localization, Function, and Human Disease
- PMID: 27766264
- PMCID: PMC5059523
- DOI: 10.1155/2016/6786245
ABC Transporter Subfamily D: Distinct Differences in Behavior between ABCD1-3 and ABCD4 in Subcellular Localization, Function, and Human Disease
Abstract
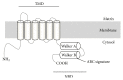
ATP-binding cassette (ABC) transporters are one of the largest families of membrane-bound proteins and transport a wide variety of substrates across both extra- and intracellular membranes. They play a critical role in maintaining cellular homeostasis. To date, four ABC transporters belonging to subfamily D have been identified. ABCD1-3 and ABCD4 are localized to peroxisomes and lysosomes, respectively. ABCD1 and ABCD2 are involved in the transport of long and very long chain fatty acids (VLCFA) or their CoA-derivatives into peroxisomes with different substrate specificities, while ABCD3 is involved in the transport of branched chain acyl-CoA into peroxisomes. On the other hand, ABCD4 is deduced to take part in the transport of vitamin B12 from lysosomes into the cytosol. It is well known that the dysfunction of ABCD1 results in X-linked adrenoleukodystrophy, a severe neurodegenerative disease. Recently, it is reported that ABCD3 and ABCD4 are responsible for hepatosplenomegaly and vitamin B12 deficiency, respectively. In this review, the targeting mechanism and physiological functions of the ABCD transporters are summarized along with the related disease.
Figures

References
-
- Kamijo K., Taketani S., Yokota S., Osumi T., Hashimoto T. The 70-kDa peroxisomal membrane protein is a member of the Mdr (P-glycoprotein)-related ATP-binding protein superfamily. The Journal of Biological Chemistry. 1990;265(8):4534–4540. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

