Alemtuzumab treatment alters circulating innate immune cells in multiple sclerosis
- PMID: 27766281
- PMCID: PMC5063395
- DOI: 10.1212/NXI.0000000000000289
Alemtuzumab treatment alters circulating innate immune cells in multiple sclerosis
Abstract
Objective: To characterize changes in myeloid and lymphoid innate immune cells in patients with relapsing-remitting multiple sclerosis (MS) during a 6-month follow-up after alemtuzumab treatment.
Methods: Circulating innate immune cells including myeloid cells and innate lymphoid cells (ILCs) were analyzed before and 6 and 12 months after onset of alemtuzumab treatment. Furthermore, a potential effect on granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-23 production by myeloid cells and natural killer (NK) cell cytolytic activity was determined.
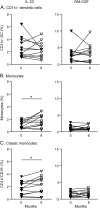
Results: In comparison to CD4+ T lymphocytes, myeloid and lymphoid innate cell subsets of patients with MS expressed significantly lower amounts of CD52 on their cell surface. Six months after CD52 depletion, numbers of circulating plasmacytoid dendritic cells (DCs) and conventional DCs were reduced compared to baseline. GM-CSF and IL-23 production in DCs remained unchanged. Within the ILC compartment, the subset of CD56bright NK cells specifically expanded under alemtuzumab treatment, but their cytolytic activity did not change.
Conclusions: Our findings demonstrate that 6 months after alemtuzumab treatment, specific DC subsets are reduced, while CD56bright NK cells expanded in patients with MS. Thus, alemtuzumab specifically restricts the DC compartment and expands the CD56bright NK cell subset with potential immunoregulatory properties in MS. We suggest that remodeling of the innate immune compartment may promote long-term efficacy of alemtuzumab and preserve immunocompetence in patients with MS.
Figures




References
-
- Hale G, Xia MQ, Tighe HP, Dyer MJ, Waldmann H. The CAMPATH-1 antigen (CDw52). Tissue Antigens 1990;35:118–127. - PubMed
-
- Mone AP, Cheney C, Banks AL, et al. . Alemtuzumab induces caspase-independent cell death in human chronic lymphocytic leukemia cells through a lipid raft-dependent mechanism. Leukemia 2006;20:272–279. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
