Epithelial Regeneration After Gastric Ulceration Causes Prolonged Cell-Type Alterations
- PMID: 27766298
- PMCID: PMC5042868
- DOI: 10.1016/j.jcmgh.2016.05.005
Epithelial Regeneration After Gastric Ulceration Causes Prolonged Cell-Type Alterations
Abstract
Background & aims: The peptic ulcer heals through a complex process, although the ulcer relapse often occurs several years later after healing. Our hypothesis is that even after visual evidence of healing of gastric ulceration, the regenerated epithelium is aberrant for an extended interval, increasing susceptibility of the regenerated epithelium to damage and further diseases.
Methods: Gastric ulcers were induced in mice by serosal topical application of acetic acid.
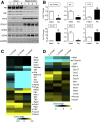
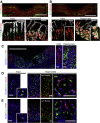
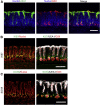
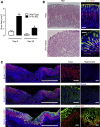
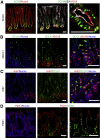
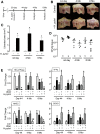
Results: Gastric ulcers induced by acetic acid visually healed within 30 days. However, regenerated epithelial architecture was poor. The gene profile of regenerated tissue was abnormal, indicating increased stem/progenitor cells, deficient differentiated gastric cell types, and deranged cell homeostasis. Despite up-regulation of PDX1 in the regenerated epithelium, no mature antral cell type was observed. Four months after healing, the regenerated epithelium lacks parietal cells, trefoil factor 2 (TFF2) and (sex-determining region Y)-box 9 (SOX9) remain up-regulated deep in the gastric gland, and the Na/H exchanger 2 (a TFF2 effector in gastric healing) remains down-regulated. Gastric ulcer healing was strongly delayed in TFF2 knockout mice, and re-epithelialization was accompanied with mucous metaplasia. After Helicobacter pylori inoculum 30 days after ulceration, we observed that the gastric ulcer selectively relapses at the same site where it originally was induced. Follow-up evaluation at 8 months showed that the relapsed ulcer was not healed in H pylori-infected tissues.
Conclusions: These findings show that this macroscopically regenerated epithelium has prolonged abnormal cell distribution and is differentially susceptible to subsequent damage by H pylori.
Keywords: CXCR4, C-X-C chemokine receptor type 4; DCLK1, doublecortin-like kinase 1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GIF, gastric intrinsic factor; GSII, Griffonia simplicifolia lectin II; Gastric Ulcer Healing; H pylori; HK-ATPase, hydrogen potassium exchanger adenosine triphosphatase; KO, knockout; Lgr5, Leucine-rich repeat-containing G protein-coupled receptor5; MUC, Mucin; Metaplasia; NHE2; NHE2, sodium hydrogen exchanger 2; PCR, polymerase chain reaction; PDX1, pancreatic and duodenal homeobox 1; SOX2, (sex-determining region Y)-box 2; SOX9; SPEM, spasmolytic polypeptide-expressing metaplasia; TFF2; TFF2, trefoil factor 2; UEA-1, ulex europaeus; WT, wild type; cDNA, complementary DNA; mRNA, messenger RNA.
Conflict of interest statement
The remaining authors declare no conflict of interest.
Figures









References
-
- Okabe S., Amagase K. An overview of acetic acid ulcer models–the history and state of the art of peptic ulcer research. Biol Pharm Bull. 2005;28:1321–1341. - PubMed
-
- Wallace J.L. Prostaglandins, NSAIDs, and gastric mucosal protection: why doesn't the stomach digest itself? Physiol Rev. 2008;88:1547–1565. - PubMed
-
- Tarnawski A., Stachura J., Krause W.J. Quality of gastric ulcer healing: a new, emerging concept. J Clin Gastroenterol. 1991;13(Suppl 1):S42–S47. - PubMed
-
- Take S., Mizuno M., Ishiki K. The effect of eradicating Helicobacter pylori on the development of gastric cancer in patients with peptic ulcer disease. Am J Gastroenterol. 2005;100:1037–1042. - PubMed
-
- Take S., Mizuno M., Ishiki K. Seventeen-year effects of eradicating Helicobacter pylori on the prevention of gastric cancer in patients with peptic ulcer; a prospective cohort study. J Gastroenterol. 2015;50:638–644. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

