Cross-Linking GPVI-Fc by Anti-Fc Antibodies Potentiates Its Inhibition of Atherosclerotic Plaque- and Collagen-Induced Platelet Activation
- PMID: 27766315
- PMCID: PMC5063538
- DOI: 10.1016/j.jacbts.2016.03.008
Cross-Linking GPVI-Fc by Anti-Fc Antibodies Potentiates Its Inhibition of Atherosclerotic Plaque- and Collagen-Induced Platelet Activation
Abstract
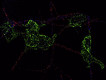
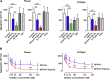
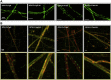
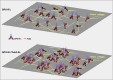
To enhance the antithrombotic properties of recombinant glycoprotein VI fragment crystallizable (GPVI-Fc), the authors incubated GPVI-Fc with anti-human Fc antibodies to cross-link the Fc tails of GPVI-Fc. Cross-linking potentiated the inhibition of human plaque- and collagen-induced platelet aggregation by GPVI-Fc under static and flow conditions without increasing bleeding time in vitro. Cross-linking with anti-human-Fc Fab2 was even superior to anti-human-Fc immunoglobulin G (IgG). Advanced optical imaging revealed a continuous sheath-like coverage of collagen fibers by cross-linked GPVI-Fc complexes. Cross-linking of GPVI into oligomeric complexes provides a new, highly effective, and probably safe antithrombotic treatment as it suppresses platelet GPVI-plaque interaction selectively at the site of acute atherothrombosis.
Keywords: Fc, fragment crystallizable; GPVI, glycoprotein VI; IgG, immunoglobulin G; PE, phycoerythrin; SIM, structured illumination microscopy; STED, stimulated emission depletion; XL, cross-linked; antithrombotic; atherothrombosis; glycoprotein VI; plaque rupture.
Figures









References
-
- Fuster V., Moreno P.R., Fayad Z.A., Corti R., Badimon J.J. Atherothrombosis and high-risk plaque: part I: evolving concepts. J Am Coll Cardiol. 2005;46:937–954. - PubMed
-
- Badimon L., Vilahur G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J Intern Med. 2014;276:618–632. - PubMed
-
- Penz S., Reininger A.J., Brandl R. Human atheromatous plaques stimulate thrombus formation by activating platelet glycoprotein VI. FASEB J. 2005;19:898–909. - PubMed
-
- Reininger A.J., Bernlochner I., Penz S.M. A 2-step mechanism of arterial thrombus formation induced by human atherosclerotic plaques. J Am Coll Cardiol. 2010;55:1147–1158. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

