Reactions of HDDA-Derived Benzynes with Perylenes: Rapid Construction of Polycyclic Aromatic Compounds
- PMID: 27766886
- PMCID: PMC5107110
- DOI: 10.1021/acs.orglett.6b02878
Reactions of HDDA-Derived Benzynes with Perylenes: Rapid Construction of Polycyclic Aromatic Compounds
Abstract
Benzynes produced by the thermal cycloisomerization of tetrayne substrates [i.e., by the hexadehydro-Diels-Alder (HDDA) reaction] react with perylenes to produce novel naphthoperylene derivatives. Cyclic voltammetry and absorption and emission properties of these compounds are described. DFT studies shed additional light on the dearomatization that accompanies the reaction as well as some of the spectroscopic behavior.
Figures


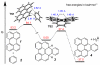
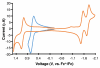
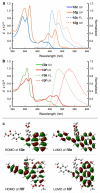
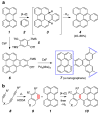

References
-
- Harvey RG. Polycyclic aromatic hydrocarbons. Wiley-VCH; New York: 1997.
- Siegel JS, Wu Y-T, Bettinger HF. Topics in Current Chemistry, 350. Springer Verlag; Heidelberg: 2014. Polyarenes I.
- Siegel JS, Wu Y-T, Bettinger HF. Topics in Current Chemistry. Vol. 349. Springer Verlag; Heidelberg: 2014. Polyarenes II.
- Stepien M, Gonka E, Zyla M, Sputta N. Chem. Rev. ASAP; 2016. - PubMed
-
- Zhao YS, editor. Organic Nanophotonics: Fundamentals and Applications. Nano-Optics and Nanophotonics. Springer; Berlin: 2014.
-
- Parker TC, Marder SR. Synthetic methods in organic electronic and photonic materials: A practical guide. Royal Society of Chemistry; Cambridge, UK: 2015.
-
- Markiewicz JT, Wudl F. ACS Appl. Mater. Interfaces. 2015;7:28063–28085. - PubMed
-
- Stork G, Matsuda K. 3,364,275 U.S. Patent. 1968
- Fort EH, Scott LT. Tetrahedron Lett. 2011;52:2051–2053.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

