Tumor-targeted delivery of a C-terminally truncated FADD (N-FADD) significantly suppresses the B16F10 melanoma via enhancing apoptosis
- PMID: 27767039
- PMCID: PMC5073321
- DOI: 10.1038/srep34178
Tumor-targeted delivery of a C-terminally truncated FADD (N-FADD) significantly suppresses the B16F10 melanoma via enhancing apoptosis
Abstract
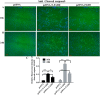
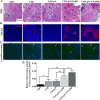
Fas-associated protein with death domain (FADD), a pivotal adaptor protein transmitting apoptotic signals, is indispensable for the induction of extrinsic apoptosis. However, overexpression of FADD can form large, filamentous aggregates, termed death effector filaments (DEFs) by self-association and initiate apoptosis independent of receptor cross-linking. A mutant of FADD, which is truncated of the C-terminal tail (m-FADD, 182-205 aa) named N-FADD (m-FADD, 1-181 aa), can dramatically up-regulate the strength of FADD self-association and increase apoptosis. In this study, it was found that over-expression of FADD or N-FADD caused apoptosis of B16F10 cells in vitro, even more, N-FADD showed a more potent apoptotic effect than FADD. Meanwhile, Attenuated Salmonella Typhimurium strain VNP20009 was engineered to express FADD or N-FADD under the control of a hypoxia-induced NirB promoter and each named VNP-pN-FADD and VNP-pN-N-FADD. The results showed both VNP-pN-FADD and VNP-pN-N-FADD delayed tumor growth in B16F10 mice model, while VNP-pN-N-FADD suppressed melanoma growth more significantly than VNP-pN-FADD. Additionally, VNP-pN-FADD and VNP-pN-N-FADD induced apoptosis of tumor cells by activating caspase-dependent apoptotic pathway. Our results show that N-FADD is a more potent apoptotic inducer and VNP20009-mediated targeted expression of N-FADD provides a possible cancer gene therapeutic approach for the treatment of melanoma.
Figures




Similar articles
-
Bacteria-mediated tumor-targeted delivery of tumstatin (54-132) significantly suppresses tumor growth in mouse model by inhibiting angiogenesis and promoting apoptosis.Front Med. 2022 Dec;16(6):873-882. doi: 10.1007/s11684-022-0925-2. Epub 2022 Sep 24. Front Med. 2022. PMID: 36152127
-
Tumor-specific delivery of histidine-rich glycoprotein suppresses tumor growth and metastasis by anti-angiogenesis and vessel normalization.Curr Gene Ther. 2014;14(2):75-85. doi: 10.2174/1566523214666140305223912. Curr Gene Ther. 2014. PMID: 24606115
-
Tumor-specifically hypoxia-induced therapy of SPRY1/2 displayed differential therapeutic efficacy for melanoma.Am J Cancer Res. 2015 Jan 15;5(2):792-801. eCollection 2015. Am J Cancer Res. 2015. PMID: 25973316 Free PMC article.
-
Salmonella-mediated tumor-targeting TRAIL gene therapy significantly suppresses melanoma growth in mouse model.Cancer Sci. 2012 Feb;103(2):325-33. doi: 10.1111/j.1349-7006.2011.02147.x. Epub 2011 Dec 15. Cancer Sci. 2012. PMID: 22054098
-
FADD in Cancer: Mechanisms of Altered Expression and Function, and Clinical Implications.Cancers (Basel). 2019 Sep 29;11(10):1462. doi: 10.3390/cancers11101462. Cancers (Basel). 2019. PMID: 31569512 Free PMC article. Review.
Cited by
-
Cell-Penetrable Peptide-Conjugated FADD Induces Apoptosis and Regulates Inflammatory Signaling in Cancer Cells.Int J Mol Sci. 2020 Sep 19;21(18):6890. doi: 10.3390/ijms21186890. Int J Mol Sci. 2020. PMID: 32961826 Free PMC article.
-
A Novel Naphthyridine Derivative, 3u, Induces Necroptosis at Low Concentrations and Apoptosis at High Concentrations in Human Melanoma A375 Cells.Int J Mol Sci. 2018 Sep 29;19(10):2975. doi: 10.3390/ijms19102975. Int J Mol Sci. 2018. PMID: 30274263 Free PMC article.
-
Mining prognostic markers of Asian hepatocellular carcinoma patients based on the apoptosis-related genes.BMC Cancer. 2021 Feb 18;21(1):175. doi: 10.1186/s12885-021-07886-6. BMC Cancer. 2021. PMID: 33602168 Free PMC article.
-
FADD as a key molecular player in cancer progression.Mol Med. 2022 Nov 8;28(1):132. doi: 10.1186/s10020-022-00560-y. Mol Med. 2022. PMID: 36348274 Free PMC article. Review.
-
Salmonella enterica and outer membrane vesicles are current and future options for cancer treatment.Front Cell Infect Microbiol. 2023 Dec 5;13:1293351. doi: 10.3389/fcimb.2023.1293351. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 38116133 Free PMC article. Review.
References
-
- Mihic L. L., Bulat V., Situm M., Krolo I. & Seserko A. The role of apoptosis in the pathogenesis of malignant melanoma. Collegium antropologicum 34 Suppl 2, 303–306 (2010). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

