Direct Analysis and Quantification of Metaldehyde in Water using Reactive Paper Spray Mass Spectrometry
- PMID: 27767044
- PMCID: PMC5073298
- DOI: 10.1038/srep35643
Direct Analysis and Quantification of Metaldehyde in Water using Reactive Paper Spray Mass Spectrometry
Abstract
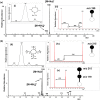
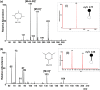
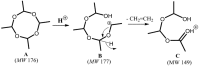
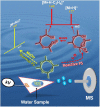
Metaldehyde is extensively used worldwide as a contact and systemic molluscicide for controlling slugs and snails in a wide range of agricultural and horticultural crops. Contamination of surface waters due to run-off, coupled with its moderate solubility in water, has led to increased concentration of the pesticide in the environment. In this study, for the first time, rapid analysis (<~1 minute) of metaldehyde residues in water is demonstrated using paper spray mass spectrometry (PS-MS). The observed precursor molecular ions of metaldehyde were confirmed from tandem mass spectrometry (MS/MS) experiments by studying the fragmentation patterns produced via collision-induced dissociation. The signal intensity ratios of the most abundant MS/MS transitions for metaldehyde (177 → 149 for protonated ion) and atrazine (221 → 179) were found to be linear in the range 0.01 to 5 ng/mL. Metaldehyde residues were detectable in environmental water samples at low concentration (LOD < 0.1 ng/mL using reactive PS-MS), with a relative standard deviation <10% and an R2 value >0.99, without any pre-concentration/separation steps. This result is of particular importance for environmental monitoring and water quality analysis providing a potential means of rapid screening to ensure safe drinking water.
Figures


Similar articles
-
An improved method for measuring metaldehyde in surface water using liquid chromatography tandem mass spectrometry.MethodsX. 2016 Mar 10;3:188-94. doi: 10.1016/j.mex.2016.03.004. eCollection 2016. MethodsX. 2016. PMID: 27054094 Free PMC article.
-
Comparison of different monitoring methods for the measurement of metaldehyde in surface waters.Environ Monit Assess. 2019 Jan 15;191(2):75. doi: 10.1007/s10661-019-7221-x. Environ Monit Assess. 2019. PMID: 30648204 Free PMC article.
-
Determination of metaldehyde in human serum by headspace solid-phase microextraction and gas chromatography-mass spectrometry.J Chromatogr B Analyt Technol Biomed Life Sci. 2008 Nov 15;875(2):573-6. doi: 10.1016/j.jchromb.2008.10.002. Epub 2008 Oct 9. J Chromatogr B Analyt Technol Biomed Life Sci. 2008. PMID: 18945651
-
Screening of synthetic PDE-5 inhibitors and their analogues as adulterants: analytical techniques and challenges.J Pharm Biomed Anal. 2014 Jan;87:176-90. doi: 10.1016/j.jpba.2013.04.037. Epub 2013 May 6. J Pharm Biomed Anal. 2014. PMID: 23721687 Review.
-
Collisional activation of peptide ions in FT-ICR mass spectrometry.Mass Spectrom Rev. 2003 May-Jun;22(3):158-81. doi: 10.1002/mas.10041. Mass Spectrom Rev. 2003. PMID: 12838543 Review.
Cited by
-
A Novel Integrated APCI and MPT Ionization Technique as Online Sensor for Trace Pesticides Detection.Sensors (Basel). 2022 Feb 25;22(5):1816. doi: 10.3390/s22051816. Sensors (Basel). 2022. PMID: 35270963 Free PMC article.
-
Accelerated nucleophilic substitution reactions of dansyl chloride with aniline under ambient conditions via dual-tip reactive paper spray.Sci Rep. 2020 Dec 9;10(1):21504. doi: 10.1038/s41598-020-78133-4. Sci Rep. 2020. PMID: 33299034 Free PMC article.
-
Novel Selectivity-Based Forensic Toxicological Validation of a Paper Spray Mass Spectrometry Method for the Quantitative Determination of Eight Amphetamines in Whole Blood.J Am Soc Mass Spectrom. 2017 Dec;28(12):2665-2676. doi: 10.1007/s13361-017-1790-0. Epub 2017 Sep 6. J Am Soc Mass Spectrom. 2017. PMID: 28879579
-
Rapid Scotch Whisky Analysis and Authentication using Desorption Atmospheric Pressure Chemical Ionisation Mass Spectrometry.Sci Rep. 2019 May 29;9(1):7994. doi: 10.1038/s41598-019-44456-0. Sci Rep. 2019. PMID: 31142757 Free PMC article.
-
Furo[3,2-c]coumarin-derived Fe3+ Selective Fluorescence Sensor: Synthesis, Fluorescence Study and Application to Water Analysis.Sci Rep. 2020 May 4;10(1):7421. doi: 10.1038/s41598-020-63262-7. Sci Rep. 2020. PMID: 32366859 Free PMC article.
References
-
- Caloni F., Cortinovis C., Rivolta M. & Davanzo F. Suspected poisoning of domestic animals by pesticides. Sci. Total Environ. 539, 331–336 (2016). - PubMed
-
- Bleakley C., Ferrie E., Collum N. & Burke L. Self-poisoning with metaldehyde. Emerg. Med. J. 25, 381–382 (2008). - PubMed
-
- Stuart M., Lapworth D., Crane E. & Hart A. Review of risk from potential emerging contaminants in UK groundwater. Sci. Total Environ. 416, 1–21 (2012). - PubMed
-
- Richardson S. D. & Ternes T. A. Water analysis: emerging contaminants and current issues. Anal. Chem. 86, 2813–2848 (2014). - PubMed
-
- Richardson S. D. & Postigo C. In Emerging organic contaminants and human health Springer-Verlag: Heidelberg, Berlin, pp 93–1376 (2012).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

