Inhibitory phosphorylation of Cdk1 mediates prolonged prophase I arrest in female germ cells and is essential for female reproductive lifespan
- PMID: 27767095
- PMCID: PMC5099868
- DOI: 10.1038/cr.2016.119
Inhibitory phosphorylation of Cdk1 mediates prolonged prophase I arrest in female germ cells and is essential for female reproductive lifespan
Abstract
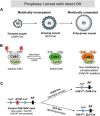
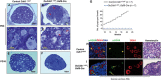
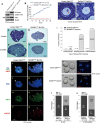
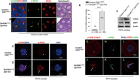
A unique feature of female germ cell development in mammals is their remarkably long arrest at the prophase of meiosis I, which lasts up to 50 years in humans. Both dormant and growing oocytes are arrested at prophase I and completely lack the ability to resume meiosis. Here, we show that the prolonged meiotic arrest of female germ cells is largely achieved via the inhibitory phosphorylation of Cdk1 (cyclin-dependent kinase 1). In two mouse models where we have introduced mutant Cdk1T14AY15F which cannot be inhibited by phosphorylation (Cdk1AF) in small meiotically incompetent oocytes, the prophase I arrest is interrupted, leading to a premature loss of female germ cells. We show that in growing oocytes, Cdk1AF leads to premature resumption of meiosis with condensed chromosomes and germinal vesicle breakdown followed by oocyte death, whereas in dormant oocytes, Cdk1AF leads to oocyte death directly, and both situations damage the ovarian reserve that maintains the female reproductive lifespan, which should be around 1 year in mice. Furthermore, interruption of the inhibitory phosphorylation of Cdk1 results in DNA damage, which is accompanied by induction of the Chk2 (checkpoint kinase 2)-p53/p63-dependent cell death pathway, which eventually causes global oocyte death. Together, our data demonstrate that the phosphorylation-mediated suppression of Cdk1 activity is one of the crucial factors that maintain the lengthy prophase arrest in mammalian female germ cells, which is essential for preserving the germ cell pool and reproductive lifespan in female mammals.
Figures
References
-
- McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev 2000; 21:200–214. - PubMed
-
- Adhikari D, Liu K. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr Rev 2009; 30:438–464. - PubMed
-
- Eppig JJ, Viveiros MM, Bivens CM, De La Fuente R. Regulation of mammalian oocyte maturation. In: Leung PC, Adashi EY, eds. The Ovary. Academic Press: Amsterdam 2004:113–129.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

