FoxO-dependent atrogenes vary among catabolic conditions and play a key role in muscle atrophy induced by hindlimb suspension
- PMID: 27767211
- PMCID: PMC5309360
- DOI: 10.1113/JP273097
FoxO-dependent atrogenes vary among catabolic conditions and play a key role in muscle atrophy induced by hindlimb suspension
Abstract
Key points: Muscle atrophy is a debilitating condition that affects a high percentage of the population with a negative impact on quality of life. Dissecting the molecular level of the atrophy process, and the similarities/dissimilarities among different catabolic conditions, is a necessary step for designing specific countermeasures to attenuate/prevent muscle loss. The FoxO family transcription factors represent one of the most important regulators of atrophy programme stimulating the expression of many atrophy-related genes. The findings of the present study clearly indicate that the signalling network controlling the atrophy programme is specific for each catabolic condition.
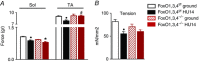
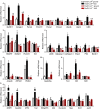
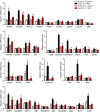
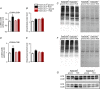
Abstract: Muscle atrophy is a complex process that is in common with many different catabolic diseases including disuse/inactivity and ageing. The signalling pathways that control the atrophy programme in the different disuse/inactivity conditions have not yet been completely dissected. The inhibition of FoxO is considered to only partially spare muscle mass after denervation. The present study aimed: (i) to determine the involvement of FoxOs in hindlimb suspension disuse model; (ii) to define whether the molecular events of protein breakdown are shared among different unloaded muscles; and finally (iii) to compare the data obtained in this model with another model of inactivity such as denervation. Both wild-type and muscle-specific FoxO1,3,4 knockout (FoxO1,3,4-/- ) mice were unloaded for 3 and 14 days and muscles were characterized by functional, morphological, biochemical and molecular assays. The data obtained show that FoxOs are required for muscle loss and force drop during unloading. Moreover, we found that FoxO-dependent atrogenes vary in different unloaded muscles and that they diverge from denervation. The findings of the present study clearly indicate that the signalling network that controls the atrophy programme is specific for each catabolic condition.
Keywords: atrogenes regulation; muscle atrophy; muscle disuse.
© 2016 The Authors. The Journal of Physiology © 2016 The Physiological Society.
Figures


Comment in
-
Master and commander? FoxO's role in muscle atrophy.J Physiol. 2017 Jul 15;595(14):4593-4594. doi: 10.1113/JP274554. Epub 2017 Jun 1. J Physiol. 2017. PMID: 28488343 Free PMC article. No abstract available.
References
-
- Blaauw B, Canato M, Agatea L, Toniolo L, Mammucari C, Masiero E, Abraham R, Sandri M, Schiaffino S & Reggiani C (2009). Inducible activation of Akt increases skeletal muscle mass and force without satellite cell activation. FASEB J 23, 3896–3905. - PubMed
-
- Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD & Glass DJ (2001). Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294, 1704–1708. - PubMed
-
- Bottinelli R & Reggiani C (2000). Human skeletal muscle fibres: molecular and functional diversity. Prog Biophys Mol Biol 73, 195–262. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

