Association of Distinct Mutational Signatures With Correlates of Increased Immune Activity in Pancreatic Ductal Adenocarcinoma
- PMID: 27768182
- PMCID: PMC5824324
- DOI: 10.1001/jamaoncol.2016.3916
Association of Distinct Mutational Signatures With Correlates of Increased Immune Activity in Pancreatic Ductal Adenocarcinoma
Abstract
Importance: Outcomes for patients with pancreatic ductal adenocarcinoma (PDAC) remain poor. Advances in next-generation sequencing provide a route to therapeutic approaches, and integrating DNA and RNA analysis with clinicopathologic data may be a crucial step toward personalized treatment strategies for this disease.
Objective: To classify PDAC according to distinct mutational processes, and explore their clinical significance.
Design, setting, and participants: We performed a retrospective cohort study of resected PDAC, using cases collected between 2008 and 2015 as part of the International Cancer Genome Consortium. The discovery cohort comprised 160 PDAC cases from 154 patients (148 primary; 12 metastases) that underwent tumor enrichment prior to whole-genome and RNA sequencing. The replication cohort comprised 95 primary PDAC cases that underwent whole-genome sequencing and expression microarray on bulk biospecimens.
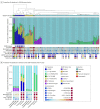
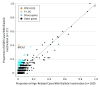
Main outcomes and measures: Somatic mutations accumulate from sequence-specific processes creating signatures detectable by DNA sequencing. Using nonnegative matrix factorization, we measured the contribution of each signature to carcinogenesis, and used hierarchical clustering to subtype each cohort. We examined expression of antitumor immunity genes across subtypes to uncover biomarkers predictive of response to systemic therapies.
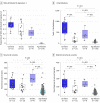
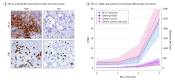
Results: The discovery cohort was 53% male (n = 79) and had a median age of 67 (interquartile range, 58-74) years. The replication cohort was 50% male (n = 48) and had a median age of 68 (interquartile range, 60-75) years. Five predominant mutational subtypes were identified that clustered PDAC into 4 major subtypes: age related, double-strand break repair, mismatch repair, and 1 with unknown etiology (signature 8). These were replicated and validated. Signatures were faithfully propagated from primaries to matched metastases, implying their stability during carcinogenesis. Twelve of 27 (45%) double-strand break repair cases lacked germline or somatic events in canonical homologous recombination genes-BRCA1, BRCA2, or PALB2. Double-strand break repair and mismatch repair subtypes were associated with increased expression of antitumor immunity, including activation of CD8-positive T lymphocytes (GZMA and PRF1) and overexpression of regulatory molecules (cytotoxic T-lymphocyte antigen 4, programmed cell death 1, and indolamine 2,3-dioxygenase 1), corresponding to higher frequency of somatic mutations and tumor-specific neoantigens.
Conclusions and relevance: Signature-based subtyping may guide personalized therapy of PDAC in the context of biomarker-driven prospective trials.
Conflict of interest statement
Figures

References
-
- National Cancer Institute, Surveillance, Epidemiology, and End Results Program. SEER Stat Fact Sheets: Pancreas Cancer. http://seer.cancer.gov/statfacts/html/pancreas.html. Accessed December 12, 2015.
-
- Kalser MH, Ellenberg SS. Pancreatic cancer: adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120(8):899-903. - PubMed
-
- Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310(14):1473-1481. - PubMed
-
- Garrido-Laguna I, Hidalgo M. Pancreatic cancer: from state-of-the-art treatments to promising novel therapies. Nat Rev Clin Oncol. 2015;12(6):319-334. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

