X-linked inhibitor of apoptosis-associated factor 1 regulates TNF receptor 1 complex stability
- PMID: 27768232
- PMCID: PMC5154952
- DOI: 10.1002/1873-3468.12467
X-linked inhibitor of apoptosis-associated factor 1 regulates TNF receptor 1 complex stability
Abstract
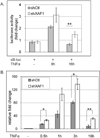
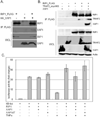
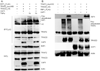
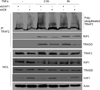
X-linked inhibitor of apoptosis (XIAP)-associated factor 1 (XAF1) is a cytokine-regulated, tumor necrosis factor (TNF) receptor-associated factor (TRAF) domain-containing protein that has a poorly defined cellular function. Here, we show that ectopically expressed XAF1 inhibits TNF-ɑ-induced NF-κB activation, whereas shRNA silencing of endogenous XAF1 augments it. Our data suggest that XAF1 may inhibit TNF-ɑ-induced NF-κB activation by disrupting the assembly of the TRADD/TRAF2/RIP1 complex (complex I) downstream of TNF receptor activation. XAF1 interacts with TRAF2 and inhibits TRAF2-dependent NF-κB activation, in part, by blocking TRAF2 polyubiquitination. Our findings also indicate that although XAF1 does not directly inhibit RIP1-dependent NF-κB activation, it binds RIP1 and disrupts RIP1/TRADD association. Our data suggest that XAF1 acts as a feedback regulator of the TNF receptor signaling pathway to suppress NF-κB activation.
Keywords: Receptor interacting protein kinase 1; XIAP associated factor 1; inflammatory response; nuclear factor-kappa B; tumor necrosis factor; tumor necrosis factor receptor-associated factor 2.
© 2016 Federation of European Biochemical Societies.
Figures




References
-
- Gruss HJ, Dower SK. Tumor necrosis factor ligand superfamily: involvement in the pathology of malignant lymphomas. Blood. 1995;85:3378–3404. - PubMed
-
- Tartaglia LA, Goeddel DV. Two TNF receptors. Immunol Today. 1992;13:151–153. - PubMed
-
- Tracey KJ, Cerami A. Tumor necrosis factor: a pleiotropic cytokine and therapeutic target. Annu Rev Med. 1994;45:491–503. - PubMed
-
- Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science. 2002;296:1634–1635. - PubMed
-
- Hsu H, Shu HB, Pan MG, Goeddel DV. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84:299–308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

