The efficacy and safety of apremilast, etanercept and placebo in patients with moderate-to-severe plaque psoriasis: 52-week results from a phase IIIb, randomized, placebo-controlled trial (LIBERATE)
- PMID: 27768242
- PMCID: PMC5363370
- DOI: 10.1111/jdv.14015
The efficacy and safety of apremilast, etanercept and placebo in patients with moderate-to-severe plaque psoriasis: 52-week results from a phase IIIb, randomized, placebo-controlled trial (LIBERATE)
Abstract
Background: Apremilast, an oral, small-molecule phosphodiesterase 4 inhibitor, has demonstrated efficacy in patients with moderate-to-severe psoriasis.
Objective: Evaluate efficacy and safety of apremilast vs. placebo in biologic-naive patients with moderate-to-severe plaque psoriasis and safety of switching from etanercept to apremilast in a phase IIIb, randomized, double-blind, placebo-controlled study (NCT01690299).
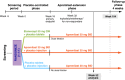
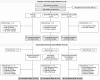
Methods: Two hundred and fifty patients were randomized to placebo (n = 84), apremilast 30 mg BID (n = 83) or etanercept 50 mg QW (n = 83) through Week 16; thereafter, all patients continued or switched to apremilast through Week 104. The primary efficacy endpoint was achievement of PASI-75 at Week 16 with apremilast vs. placebo. Secondary endpoints included achievement of PASI-75 at Week 16 with etanercept vs. placebo and improvements in other clinical endpoints vs. placebo at Week 16. Outcomes were assessed through Week 52. This study was not designed for apremilast vs. etanercept comparisons.
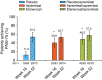
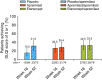
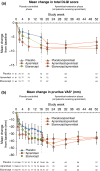
Results: At Week 16, PASI-75 achievement was greater with apremilast (39.8%) vs. placebo (11.9%; P < 0.0001); 48.2% of patients achieved PASI-75 with etanercept (P < 0.0001 vs. placebo). PASI-75 response was maintained in 47.3% (apremilast/apremilast), 49.4% (etanercept/apremilast) and 47.9% (placebo/apremilast) of patients at Week 52. Most common adverse events (≥5%) with apremilast, including nausea, diarrhoea, upper respiratory tract infection, nasopharyngitis, tension headache and headache, were mild or moderate in severity; diarrhoea and nausea generally resolved in the first month. No new safety or tolerability issues were observed through Week 52 with apremilast.
Conclusion: Apremilast demonstrated significant efficacy vs. placebo at Week 16 in biologic-naive patients with psoriasis, which was sustained over 52 weeks, and demonstrated safety consistent with the known safety profile of apremilast. Switching from etanercept to apremilast did not result in any new or clinically significant safety findings, and efficacy was maintained with apremilast through Week 52.
© 2016 The Authors. Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd on behalf of European Academy of Dermatology and Venereology.
Figures
References
-
- Reich K. The concept of psoriasis as a systemic inflammation: implications for disease management. J Eur Acad Dermatol Venereol 2012; 26(Suppl 2): 3–11. - PubMed
-
- Coimbra S, Figueiredo A, Castro E, Rocha‐Pereira P, Santos‐Silva A. The roles of cells and cytokines in the pathogenesis of psoriasis. Int J Dermatol 2012; 51: 389–398. - PubMed
-
- Taheri A, Sandoval LF, Moradi Tuchay S, Alinia H, Mansoori P, Feldman SR. Emerging treatment options for psoriasis. Psoriasis Targets Ther 2014; 4: 27–35.
-
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol 2012; 83: 1583–1590. - PubMed
-
- Schafer PH, Parton A, Capone L et al Apremilast is a selective PDE4 inhibitor with regulatory effects on innate immunity. Cell Signal 2014; 26: 2016–2029. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

