Single-Flask Multicomponent Synthesis of Highly Substituted α-Pyrones via a Sequential Enolate Arylation and Alkenylation Strategy
- PMID: 27768319
- PMCID: PMC8103787
- DOI: 10.1021/acs.orglett.6b02969
Single-Flask Multicomponent Synthesis of Highly Substituted α-Pyrones via a Sequential Enolate Arylation and Alkenylation Strategy
Abstract
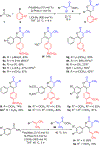
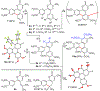
Trisubstituted α-pyrones are obtained by a Pd-catalyzed three-component, single-flask operation via an α-arylation, subsequent α-alkenylation, alkene isomerization, and dienolate lactonization. A variety of coupling components under mild conditions afforded isolated yields of up to 93% of the pyrones with complete control of regioselectivity. Metal dependence was noted for three of the steps of the pathway. Utility of the pyrone products was demonstrated by further transformations providing convenient access to polyaromatic compounds, exhibiting broad molecular diversity.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
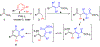

References
-
- Afarinkia K; Vinader V; Nelson TD; Posner GH Tetrahedron 1992, 48, 9111.
- Sun C-L; Fürstner A Angew. Chem., Int. Ed 2013, 52, 13071. - PubMed
- Shin H-S; Jung Y-G; Cho H-K; Park H-K; Cho C-G Org. Lett 2014, 16, 5718. - PubMed
- Okura K; Tamura R; Shigehara K; Masai E; Nakamura M; Otsuka Y; Katayama Y; Nakao Y Chem. Lett 2014, 43, 1349.
- Khatri AI; Samant SD RSC Adv 2015, 5, 2009.
- Gan P; Smith MW; Braffman NR; Snyder SA Angew. Chem., Int. Ed 2016, 55, 3625. - PubMed
-
- Goel A; Taneja G; Raghuvanshi A; Kant R; Maulik PR Org. Biomol. Chem 2013, 11, 5239. - PubMed
- Miura T; Fujioka S; Takemura N; Iwasaki H; Ozeki M; Kojima N; Yamashita M Synthesis 2014, 46, 496.
- Yeh P-P; Daniels DSB; Cordes DB; Slawin AMZ; Smith AD Org. Lett 2014, 16, 964. - PubMed
- Liu W; Zhao G Org. Biomol. Chem 2014, 12, 832. - PubMed
-
- Mochida S; Hirano K; Satoh T; Miura MJ Org. Chem 2009, 74, 6295. - PubMed
- Ackermann L; Pospech J; Graczyk K; Rauch K Org. Lett 2012, 14, 930. - PubMed
- Li Q; Yan Y; Wang X; Gong B; Tang X; Shi J; Xu HE; Yi W RSC Adv 2013, 3, 23402.
- Yu Y; Huang L; Wu W; Jiang H Org. Lett 2014, 16, 2146. - PubMed
- Moghaddam FM; Mirjafary Z; Javan MJ; Motamen S; Saeidian H Tetrahedron Lett 2014, 55, 2908.
- Manikandan R; Jeganmohan M Org. Lett 2014, 16, 652. - PubMed
- Tian P-P; Cai S-H; Liang Q-J; Zhou X-Y; Xu Y-H; Loh T-P Org. Lett 2015, 17, 1636. - PubMed
- Preindl J; Jouvin K; Laurich D; Seidel G; Fürstner A Chem. -Eur. J 2016, 22, 237. - PubMed
- Faizi DJ; Issaian A; Davis AJ; Blum SA J. Am. Chem. Soc 2016, 138, 2126. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

