Is senescence-associated β-galactosidase a marker of neuronal senescence?
- PMID: 27768595
- PMCID: PMC5348379
- DOI: 10.18632/oncotarget.12752
Is senescence-associated β-galactosidase a marker of neuronal senescence?
Abstract
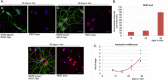
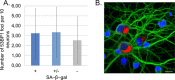
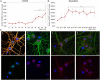
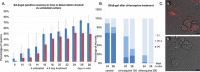
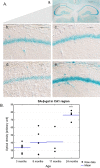
One of the features of cellular senescence is the activity of senescence-associated- β-galactosidase (SA-β-gal). The main purpose of this study was to evaluate this marker of senescence in aging neurons. We found that cortical neurons exhibited noticeable SA-β-gal activity quite early in culture. Many SA-β-gal-positive neurons were negative for another canonical marker of senescence, namely, double-strand DNA breaks (DSBs). Moreover, DDR signalling triggered by low doses of doxorubicin did not accelerate the appearance of neuronal SA-β-gal. In vivo, we observed pronounced induction of SA-β-gal activity in the hippocampus of 24-month-old mice, which is consistent with previous findings and supports the view that at this advanced age neurons developed a senescence-like phenotype. Surprisingly however, relatively high SA-β-gal activity, probably unrelated to the senescence process, was also observed in much younger, 3-month-old mice. In conclusion, we propose that SA-β-gal activity in neurons cannot be attributed uniquely to cell senescence either in vitro or in vivo. Additionally, we showed induction of REST protein in aging neurons in long-term culture and we propose that REST could be a marker of neuronal senescence in vitro.
Keywords: DNA damage response; SA-β-galactosidase; aging; neurons; senescence.
Conflict of interest statement
No competing interests declared.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

