Evaporimeter and Bubble-Imaging Measures of Sweat Gland Secretion Rates
- PMID: 27768743
- PMCID: PMC5074501
- DOI: 10.1371/journal.pone.0165254
Evaporimeter and Bubble-Imaging Measures of Sweat Gland Secretion Rates
Abstract
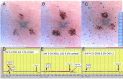
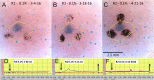
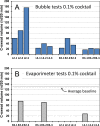
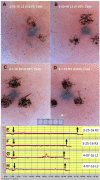
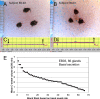
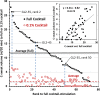
Beta-adrenergically-stimulated sweat rates determined by evaporimetry or by sweat bubble imaging are useful for measuring CFTR function because they provide a near-linear readout across almost the full range of CFTR function. They differentiate cystic fibrosis (CF) subjects from CF carriers and carriers from controls. However, evaporimetry, unlike bubble imaging, appears to be unable to detect improved levels of CFTR function in G551D subjects taking the CFTR modulator ivacaftor. Here, we quantify the sensitivity of evaporimetry and bubble imaging methods for assessing low levels of CFTR-dependent sweat rates. To establish sensitivity, we did dose-ranging studies using intradermally injected [cAMP]i-elevating cocktails. We reduced isoproterenol/aminophylline levels while maintaining a high level of atropine to block muscarinic elevation of [Ca2+]i. We stimulated the same sets of glands for both assays and recorded responses for 20 min. In response to a 3-log dilution of the stimulating cocktail (0.1%), bubble responses were detected in 12/12 tests (100%), with 49% ± 3% of glands secreting to produce an aggregate volume of 598 nl across the 12, 20-min tests. This was ~5% of the response to full cocktail. Evaporimetry detected responses in 3/12 (25%) tests with an aggregate secretion volume of 175 nl. After stimulation with a still more dilute cocktail (0.03%), bubble imaging detected 15 ± 13% of glands secreting at a rate ~0.9% of the response to full cocktail, while zero responding was seen with evaporimetry. The bubble imaging method detected secretion down to aggregate rates of <0.2 nl/(cm2·min), or ~1/30th of the average basal transepithelial water loss (TEWL) in the test subject of 4 g/m2·hr or 6.7 nl/(cm2·min). The increased sensitivity of bubble imaging may be required to detect small but physiologically important increases in secretion rates produced by CFTR modulators.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245(4922):1066–73. . - PubMed
-
- Di Sant'Agnese P, Darling RC, Perara GA, Shea E. Abnormal electrolyte composition of sweat in cystic fibrosis of the pancreas. AMA Am J Dis Child. 1953;86(5):618–9; discussion, 9. Epub 1953/11/01. . - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

