Inactivation of Tsc2 in Mesoderm-Derived Cells Causes Polycystic Kidney Lesions and Impairs Lung Alveolarization
- PMID: 27768862
- PMCID: PMC5225296
- DOI: 10.1016/j.ajpath.2016.08.013
Inactivation of Tsc2 in Mesoderm-Derived Cells Causes Polycystic Kidney Lesions and Impairs Lung Alveolarization
Abstract
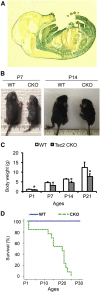
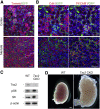
The tuberous sclerosis complex (TSC) proteins are critical negative regulators of the mammalian/mechanistic target of rapamycin complex 1 pathway. Germline mutations of TSC1 or TSC2 cause TSC, affecting multiple organs, including the kidney and lung, and causing substantial morbidity and mortality. The mechanisms of organ-specific disease in TSC remain incompletely understood, and the impact of TSC inactivation on mesenchymal lineage cells has not been specifically studied. We deleted Tsc2 specifically in mesoderm-derived mesenchymal cells of multiple organs in mice using the Dermo1-Cre driver. The Dermo1-Cre-driven Tsc2 conditional knockout mice had body growth retardation and died approximately 3 weeks after birth. Significant phenotypes were observed in the postnatal kidney and lung. Inactivation of Tsc2 in kidney mesenchyme caused polycystic lesions starting from the second week of age, with increased cell proliferation, tubular epithelial hyperplasia, and epithelial-mesenchymal transition. In contrast, Tsc2 deletion in lung mesenchyme led to decreased cell proliferation, reduced postnatal alveolarization, and decreased differentiation with reduced numbers of alveolar myofibroblast and type II alveolar epithelial cells. Two major findings thus result from this model: inactivation of Tsc2 in mesoderm-derived cells causes increased cell proliferation in the kidneys but reduced proliferation in the lungs, and inactivation of Tsc2 in mesoderm-derived cells causes epithelial-lined renal cysts. Therefore, Tsc2-mTOR signaling in mesenchyme is essential for the maintenance of renal structure and for lung alveolarization.
Copyright © 2016 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Curatolo P., Maria B.L. Tuberous sclerosis. Handb Clin Neurol. 2013;111:323–331. - PubMed
-
- Henske E.P., Jóźwiak S., Kingswood J.C., Sampson J.R., Thiele E.A. Tuberous sclerosis complex. Nat Rev Dis Primers. 2016;2:16035. - PubMed
-
- Hayashi T., Kumasaka T., Mitani K., Yao T., Suda K., Seyama K. Loss of heterozygosity on tuberous sclerosis complex genes in multifocal micronodular pneumocyte hyperplasia. Mod Pathol. 2010;23:1251–1260. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

