Rickettsia Sca4 Reduces Vinculin-Mediated Intercellular Tension to Promote Spread
- PMID: 27768890
- PMCID: PMC5097866
- DOI: 10.1016/j.cell.2016.09.023
Rickettsia Sca4 Reduces Vinculin-Mediated Intercellular Tension to Promote Spread
Abstract
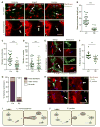
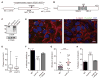
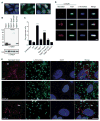
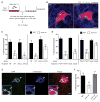
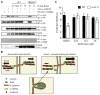
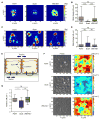
Spotted fever group (SFG) rickettsiae are human pathogens that infect cells in the vasculature. They disseminate through host tissues by a process of cell-to-cell spread that involves protrusion formation, engulfment, and vacuolar escape. Other bacterial pathogens rely on actin-based motility to provide a physical force for spread. Here, we show that SFG species Rickettsia parkeri typically lack actin tails during spread and instead manipulate host intercellular tension and mechanotransduction to promote spread. Using transposon mutagenesis, we identified surface cell antigen 4 (Sca4) as a secreted effector of spread that specifically promotes protrusion engulfment. Sca4 interacts with the cell-adhesion protein vinculin and blocks association with vinculin's binding partner, α-catenin. Using traction and monolayer stress microscopy, we show that Sca4 reduces vinculin-dependent mechanotransduction at cell-cell junctions. Our results suggest that Sca4 relieves intercellular tension to promote protrusion engulfment, which represents a distinctive strategy for manipulating cytoskeletal force generation to enable spread.
Keywords: actin-based motility; cell-to-cell spread; cytoskeleton; host-pathogen interactions; intercellular tension; listeria monocytogenes; mechanotransduction; rickettsia parkeri; sca4; vinculin.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures

Comment in
-
Bacterial pathogenesis: Rickettsia releases the tension.Nat Rev Microbiol. 2016 Dec;14(12):728-729. doi: 10.1038/nrmicro.2016.166. Epub 2016 Nov 7. Nat Rev Microbiol. 2016. PMID: 27818498 No abstract available.
-
Rickettsia Evades a Tense Situation.Cell Host Microbe. 2016 Nov 9;20(5):549-550. doi: 10.1016/j.chom.2016.10.017. Cell Host Microbe. 2016. PMID: 27832582
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

