Genomic Nucleosome Organization Reconstituted with Pure Proteins
- PMID: 27768892
- PMCID: PMC5240917
- DOI: 10.1016/j.cell.2016.09.045
Genomic Nucleosome Organization Reconstituted with Pure Proteins
Abstract
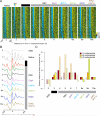
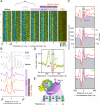
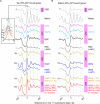
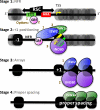
Chromatin remodelers regulate genes by organizing nucleosomes around promoters, but their individual contributions are obfuscated by the complex in vivo milieu of factor redundancy and indirect effects. Genome-wide reconstitution of promoter nucleosome organization with purified proteins resolves this problem and is therefore a critical goal. Here, we reconstitute four stages of nucleosome architecture using purified components: yeast genomic DNA, histones, sequence-specific Abf1/Reb1, and remodelers RSC, ISW2, INO80, and ISW1a. We identify direct, specific, and sufficient contributions that in vivo observations validate. First, RSC clears promoters by translating poly(dA:dT) into directional nucleosome removal. Second, partial redundancy is recapitulated where INO80 alone, or ISW2 at Abf1/Reb1sites, positions +1 nucleosomes. Third, INO80 and ISW2 each align downstream nucleosomal arrays. Fourth, ISW1a tightens the spacing to canonical repeat lengths. Such a minimal set of rules and proteins establishes core mechanisms by which promoter chromatin architecture arises through a blend of redundancy and specialization.
Keywords: Abf1; INO80; Isw; RSC; Reb1; Saccharomyces cerevisiae; chromatin; general regulatory factors (GRFs); in vitro reconstitution; nucleosome positioning and remodeling.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures



Comment in
-
Stringing Nucleosome Necklaces in the Yeast Genome.Cell. 2016 Oct 20;167(3):600-601. doi: 10.1016/j.cell.2016.10.009. Cell. 2016. PMID: 27768882
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

