Toxic PR Poly-Dipeptides Encoded by the C9orf72 Repeat Expansion Target LC Domain Polymers
- PMID: 27768897
- PMCID: PMC5076566
- DOI: 10.1016/j.cell.2016.10.003
Toxic PR Poly-Dipeptides Encoded by the C9orf72 Repeat Expansion Target LC Domain Polymers
Abstract
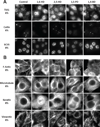
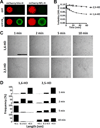
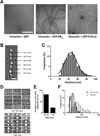
Two complementary approaches were used in search of the intracellular targets of the toxic PR poly-dipeptide encoded by the repeat sequences expanded in the C9orf72 form of amyotrophic lateral sclerosis. The top categories of PRn-bound proteins include constituents of non-membrane invested cellular organelles and intermediate filaments. PRn targets are enriched for the inclusion of low complexity (LC) sequences. Evidence is presented indicating that LC sequences represent the direct target of PRn binding and that interaction between the PRn poly-dipeptide and LC domains is polymer-dependent. These studies indicate that PRn-mediated toxicity may result from broad impediments to the dynamics of cell structure and information flow from gene to message to protein.
Keywords: 1,6-hexanediol; C9orf72; amyloid-like polymers; cellular puncta not invested by surrounding membranes; intermediate filaments; labile; low complexity sequence polymers; toxic PRn and GRn poly-dipeptides.
Published by Elsevier Inc.
Figures



References
-
- ACD/Structure Elucidator. version 15.01. Toronto, ON, Canada: Advanced Chemistry Development, Inc.; 2015. http://www.acdlabs.com/
-
- Bergeron-Sandoval LP, Safaee N, Michnick SW. Mechanisms and Consequences of Macromolecular Phase Separation. Cell. 2016;165:1067–1079. - PubMed
-
- Brownlees J, Ackerley S, Grierson AJ, Jacobsen NJ, Shea K, Anderton BH, Leigh PN, Shaw CE, Miller CC. Charcot-Marie-Tooth disease neurofilament mutations disrupt neurofilament assembly and axonal transport. Hum Mol Genet. 2002;11:2837–2844. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

